From Red to Green Luminescence via Surface Functionalization. Effect of 2-(5-Mercaptothien-2-yl)-8-(thien-2-yl)-5-hexylthieno[3,4- c]pyrrole-4,6-dione Ligands on the Photoluminescence of Alloyed Ag-In-Zn-S Nanocrystals
- PMID: 32941018
- PMCID: PMC7586334
- DOI: 10.1021/acs.inorgchem.0c02468
From Red to Green Luminescence via Surface Functionalization. Effect of 2-(5-Mercaptothien-2-yl)-8-(thien-2-yl)-5-hexylthieno[3,4- c]pyrrole-4,6-dione Ligands on the Photoluminescence of Alloyed Ag-In-Zn-S Nanocrystals
Abstract
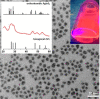
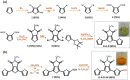
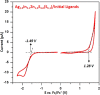
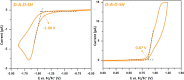
A semiconducting molecule containing a thiol anchor group, namely 2-(5-mercaptothien-2-yl)-8-(thien-2-yl)-5-hexylthieno[3,4-c]pyrrole-4,6-dione (abbreviated as D-A-D-SH), was designed, synthesized, and used as a ligand in nonstoichiometric quaternary nanocrystals of composition Ag1.0In3.1Zn1.0S4.0(S6.1) to give an inorganic/organic hybrid. Detailed NMR studies indicate that D-A-D-SH ligands are present in two coordination spheres in the organic part of the hybrid: (i) inner in which the ligand molecules form direct bonds with the nanocrystal surface and (ii) outer in which the ligand molecules do not form direct bonds with the inorganic core. Exchange of the initial ligands (stearic acid and 1-aminooctadecane) for D-A-D-SH induces a distinct change of the photoluminescence. Efficient red luminescence of nanocrystals capped with initial ligands (λmax = 720 nm, quantum yield = 67%) is totally quenched and green luminescence characteristic of the ligand appears (λmax = 508 nm, quantum yield = 10%). This change of the photoluminescence mechanism can be clarified by a combination of electrochemical and spectroscopic investigations. It can be demonstrated by cyclic voltammetry that new states appear in the hybrid as a consequence of D-A-D-SH binding to the nanocrystals surface. These states are located below the nanocrystal LUMO and above its HOMO, respectively. They are concurrent to deeper donor and acceptor states governing the red luminescence. As a result, energy transfer from the nanocrystal HOMO and LUMO levels to the ligand states takes place, leading to effective quenching of the red luminescence and appearance of the green one.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




References
LinkOut - more resources
Full Text Sources
Research Materials

