The NADase enzyme CD38: an emerging pharmacological target for systemic sclerosis, systemic lupus erythematosus and rheumatoid arthritis
- PMID: 32941246
- PMCID: PMC7807656
- DOI: 10.1097/BOR.0000000000000737
The NADase enzyme CD38: an emerging pharmacological target for systemic sclerosis, systemic lupus erythematosus and rheumatoid arthritis
Abstract
Purpose of review: Here we review recent literature on the emerging role of nicotinamide adenine dinucleotide (NAD) metabolism and its dysfunction via the enzyme CD38 in the pathogenesis of rheumatologic diseases. We evaluate the potential of targeting CD38 to ameliorate NAD-related metabolic imbalance and tissue dysfunction in the treatment of systemic sclerosis (SSc), systemic lupus erythematous (SLE), and rheumatoid arthritis (RA).
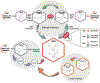
Recent findings: In this review, we will discuss emerging basic, preclinical, and human data that point to the novel role of CD38 in dysregulated NAD-homeostasis in SSc, SLE, and RA. In particular, recent studies implicate increased activity of CD38, one of the main enzymes in NAD catabolism, in the pathogenesis of persistent systemic fibrosis in SSc, and increased susceptibility of SLE patients to infections. We will also discuss recent studies that demonstrate that a cytotoxic CD38 antibody can promote clearance of plasma cells involved in the generation of RA antibodies.
Summary: Recent studies identify potential therapeutic approaches for boosting NAD to treat rheumatologic diseases including SSc, RA, and SLE, with particular attention to inhibition of CD38 enzymatic activity as a target. Key future directions in the field include the determination of the cell-type specificity and role of CD38 enzymatic activity versus CD38 structural roles in human diseases, as well as the indicators and potential side effects of CD38-targeted treatments.
Figures
References
-
- Asano Y, Varga J. Rationally-based therapeutic disease modification in systemic sclerosis: Novel strategies. Seminars in Cell & Developmental Biology. 2020;101:146–60. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

