Lack of RAC1 in macrophages protects against atherosclerosis
- PMID: 32941503
- PMCID: PMC7498073
- DOI: 10.1371/journal.pone.0239284
Lack of RAC1 in macrophages protects against atherosclerosis
Abstract
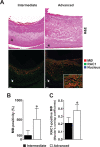
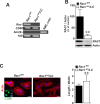
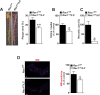
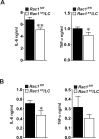
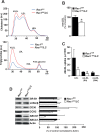
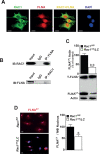
The Rho GTPase RAC1 is an important regulator of cytoskeletal dynamics, but the role of macrophage-specific RAC1 has not been explored during atherogenesis. We analyzed RAC1 expression in human carotid atherosclerotic plaques using immunofluorescence and found higher macrophage RAC1 expression in advanced plaques compared with intermediate human atherosclerotic plaques. We then produced mice with Rac1-deficient macrophages by breeding conditional floxed Rac1 mice (Rac1fl/fl) with mice expressing Cre from the macrophage-specific lysosome M promoter (LC). Atherosclerosis was studied in vivo by infecting Rac1fl/fl and Rac1fl/fl/LC mice with AdPCSK9 (adenoviral vector overexpressing proprotein convertase subtilisin/kexin type 9). Rac1fl/fl/LC macrophages secreted lower levels of IL-6 and TNF-α and exhibited reduced foam cell formation and lipid uptake. The deficiency of Rac1 in macrophages reduced the size of aortic atherosclerotic plaques in AdPCSK9-infected Rac1fl/fl/LC mice. Compare with controls, intima/media ratios, the size of necrotic cores, and numbers of CD68-positive macrophages in atherosclerotic plaques were reduced in Rac1-deficient mice. Moreover, we found that RAC1 interacts with actin-binding filamin A. Macrophages expressed increased RAC1 levels in advanced human atherosclerosis. Genetic inactivation of RAC1 impaired macrophage function and reduced atherosclerosis in mice, suggesting that drugs targeting RAC1 may be useful in the treatment of atherosclerosis.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
-
Targeting Filamin A Reduces Macrophage Activity and Atherosclerosis.Circulation. 2019 Jul 2;140(1):67-79. doi: 10.1161/CIRCULATIONAHA.119.039697. Epub 2019 Apr 24. Circulation. 2019. PMID: 31014088
-
Deletion of Rac1GTPase in the Myeloid Lineage Protects against Inflammation-Mediated Kidney Injury in Mice.PLoS One. 2016 Mar 3;11(3):e0150886. doi: 10.1371/journal.pone.0150886. eCollection 2016. PLoS One. 2016. PMID: 26939003 Free PMC article.
-
Statins Disrupt Macrophage Rac1 Regulation Leading to Increased Atherosclerotic Plaque Calcification.Arterioscler Thromb Vasc Biol. 2020 Mar;40(3):714-732. doi: 10.1161/ATVBAHA.119.313832. Epub 2020 Jan 30. Arterioscler Thromb Vasc Biol. 2020. PMID: 31996022 Free PMC article.
-
Curcuma oil attenuates accelerated atherosclerosis and macrophage foam-cell formation by modulating genes involved in plaque stability, lipid homeostasis and inflammation.Br J Nutr. 2015 Jan 14;113(1):100-13. doi: 10.1017/S0007114514003195. Epub 2014 Nov 13. Br J Nutr. 2015. PMID: 25391643
-
The anti-inflammatory vasostatin-2 attenuates atherosclerosis in ApoE-/- mice and inhibits monocyte/macrophage recruitment.Thromb Haemost. 2017 Jan 26;117(2):401-414. doi: 10.1160/TH16-06-0475. Epub 2016 Nov 10. Thromb Haemost. 2017. PMID: 27831589
Cited by
-
Targeting Rac1 for the prevention of atherosclerosis among U.S. Veterans with inflammatory bowel disease.Small GTPases. 2022 Jan;13(1):205-210. doi: 10.1080/21541248.2021.1954863. Epub 2021 Jul 28. Small GTPases. 2022. PMID: 34320903 Free PMC article.
-
Rac1 inhibition protects the kidney against kidney ischemia/reperfusion through the inhibition of macrophage migration.Korean J Physiol Pharmacol. 2023 May 1;27(3):257-265. doi: 10.4196/kjpp.2023.27.3.257. Korean J Physiol Pharmacol. 2023. PMID: 37078299 Free PMC article.
-
The potential of ARL4C and its-mediated genes in atherosclerosis and agent development.Front Pharmacol. 2025 Mar 19;16:1513340. doi: 10.3389/fphar.2025.1513340. eCollection 2025. Front Pharmacol. 2025. PMID: 40176913 Free PMC article. Review.
-
Interleukin-35 Mitigates ox-LDL-Induced Proatherogenic Effects via Modulating miRNAs Associated with Coronary Artery Disease (CAD).Cardiovasc Drugs Ther. 2023 Aug;37(4):667-682. doi: 10.1007/s10557-022-07335-x. Epub 2022 Apr 18. Cardiovasc Drugs Ther. 2023. PMID: 35435604
-
Interfering with Rac1-activation during neonatal monocyte-macrophage differentiation influences the inflammatory responses of M1 macrophages.Cell Death Dis. 2023 Sep 21;14(9):619. doi: 10.1038/s41419-023-06150-y. Cell Death Dis. 2023. PMID: 37735499 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

