Experimental Human Pneumococcal Colonization in Older Adults Is Feasible and Safe, Not Immunogenic
- PMID: 32941735
- PMCID: PMC7924584
- DOI: 10.1164/rccm.202004-1483OC
Experimental Human Pneumococcal Colonization in Older Adults Is Feasible and Safe, Not Immunogenic
Abstract
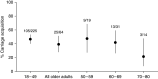
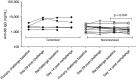
Rationale: Pneumococcal colonization is key to the pathogenesis of invasive disease but is also immunogenic in young adults, protecting against recolonization. Colonization is rarely detected in older adults, despite high rates of pneumococcal disease.Objectives: To establish experimental human pneumococcal colonization in healthy adults aged 50-84 years, to measure the immune response to pneumococcal challenge, and to assess the protective effect of prior colonization against autologous strain rechallenge.Methods: Sixty-four participants were inoculated with Streptococcus pneumoniae (serotype 6B; 80,000 cfu in each nostril). Colonization was determined by bacterial culture of nasal wash, and humoral immune responses were assessed by anticapsular and antiprotein IgG concentrations.Measurements and Main Results: Experimental colonization was established in 39% of participants (25/64) with no adverse events. Colonization occurred in 47% (9/19) of participants aged 50-59 compared with 21% (3/14) in those aged ≥70 years. Previous pneumococcal polysaccharide vaccination did not protect against colonization. Colonization did not confer serotype-specific immune boosting, with a geometric mean titer (95% confidence interval) of 2.7 μg/ml (1.9-3.8) before the challenge versus 3.0 (1.9-4.7) 4 weeks after colonization (P = 0.53). Furthermore, pneumococcal challenge without colonization led to a drop in specific antibody concentrations from 2.8 μg/ml (2.0-3.9) to 2.2 μg/ml (1.6-3.0) after the challenge (P = 0.006). Antiprotein antibody concentrations increased after successful colonization. Rechallenge with the same strain after a median of 8.5 months (interquartile range, 6.7-10.1) led to recolonization in 5/16 (31%).Conclusions: In older adults, experimental pneumococcal colonization is feasible and safe but demonstrates different immunological outcomes compared with younger adults in previous studies.
Keywords: Streptococcus pneumoniae;; elderly; human challenge models; immunity; vaccination.
Figures




Comment in
-
Experimental Model for Studies of Pneumococcal Colonization in Older Adults.Am J Respir Crit Care Med. 2021 Mar 1;203(5):539-540. doi: 10.1164/rccm.202009-3681ED. Am J Respir Crit Care Med. 2021. PMID: 33075234 Free PMC article. No abstract available.
References
-
- Welte T, Torres A, Nathwani D. Clinical and economic burden of community-acquired pneumonia among adults in Europe. Thorax. 2012;67:71–79. - PubMed
-
- Bijlsma MW, Brouwer MC, Kasanmoentalib ES, Kloek AT, Lucas MJ, Tanck MW, et al. Community-acquired bacterial meningitis in adults in the Netherlands, 2006-14: a prospective cohort study. Lancet Infect Dis. 2016;16:339–347. - PubMed
-
- Thigpen MC, Whitney CG, Messonnier NE, Zell ER, Lynfield R, Hadler JL, et al. Emerging Infections Programs Network. Bacterial meningitis in the United States, 1998-2007. N Engl J Med. 2011;364:2016–2025. - PubMed
-
- Bogaert D, De Groot R, Hermans PW. Streptococcus pneumoniae colonisation: the key to pneumococcal disease. Lancet Infect Dis. 2004;4:144–154. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

