Activate: Randomized Clinical Trial of BCG Vaccination against Infection in the Elderly
- PMID: 32941801
- PMCID: PMC7462457
- DOI: 10.1016/j.cell.2020.08.051
Activate: Randomized Clinical Trial of BCG Vaccination against Infection in the Elderly
Abstract
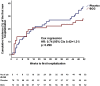
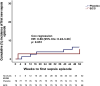
BCG vaccination in children protects against heterologous infections and improves survival independently of tuberculosis prevention. The phase III ACTIVATE trial assessed whether BCG has similar effects in the elderly. In this double-blind, randomized trial, elderly patients (n = 198) received BCG or placebo vaccine at hospital discharge and were followed for 12 months for new infections. At interim analysis, BCG vaccination significantly increased the time to first infection (median 16 weeks compared to 11 weeks after placebo). The incidence of new infections was 42.3% (95% CIs 31.9%-53.4%) after placebo vaccination and 25.0% (95% CIs 16.4%-36.1%) after BCG vaccination; most of the protection was against respiratory tract infections of probable viral origin (hazard ratio 0.21, p = 0.013). No difference in the frequency of adverse effects was found. Data show that BCG vaccination is safe and can protect the elderly against infections. Larger studies are needed to assess protection against respiratory infections, including COVID-19 (ClinicalTrials.gov NCT03296423).
Keywords: BCG; cytokines; elderly; epigenetic modifications; infection incidence; respiratory infections; trained immunity; vaccination.
Copyright © 2020 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests E.J.G.-B. has received honoraria from Abbott CH, Angelini Italy, bioMérieux Inc, InflaRx GmbH, MSD Greece, and XBiotech Inc.; independent educational grants from AbbVie, Abbott, Astellas Pharma Europe, AxisShield, bioMérieux Inc, InflaRx GmbH, ThermoFisher Brahms GmbH, and XBiotech Inc; and funding from the FrameWork 7 program HemoSpec (granted to the National and Kapodistrian University of Athens), the Horizon2020 Marie-Curie Project European Sepsis Academy (granted to the National and Kapodistrian University of Athens), and the Horizon 2020 European Grant ImmunoSep (granted to the Hellenic Institute for the Study of Sepsis). M.G.N. was supported by an ERC Advanced Grant (#833247) and a Spinoza grant of the Netherlands Organization for Scientific Research. M.G.N. is a scientific founder of TTxD. The other authors do not have any competing interests to declare.
Figures






References
-
- Antonopoulou A., Baziaka F., Tsaganos T., Raftogiannis M., Koutoukas P., Spyridaki A., Mouktaroudi M., Kotsaki A., Savva A., Georgitsi M., Giamarellos-Bourboulis E.J. Role of tumor necrosis factor gene single nucleotide polymorphisms in the natural course of 2009 influenza A H1N1 virus infection. Int. J. Infect. Dis. 2012;16:e204–e208. - PubMed
-
- Arts R.J.W., Moorlag S.J.C.F.M., Novakovic B., Li Y., Wang S.Y., Oosting M., Kumar V., Xavier R.J., Wijmenga C., Joosten L.A.B., et al. BCG vaccination protects against experimental viral infection in humans through the induction of cytokines associated with trained immunity. Cell Host Microbe. 2018;23:89–100. - PubMed
-
- Bender B.S. Infectious disease risk in the elderly. Immunol. Allergy Clin. North Am. 2003;23:57–64, vi. - PubMed
-
- Calandra T., Cohen J., International Sepsis Forum Definition of Infection in the ICU Consensus Conference The international sepsis forum consensus conference on definitions of infection in the intensive care unit. Crit. Care Med. 2005;33:1538–1548. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

