Activation of Cannabinoid Receptor 2 Prevents Colitis-Associated Colon Cancer through Myeloid Cell De-activation Upstream of IL-22 Production
- PMID: 32942172
- PMCID: PMC7501437
- DOI: 10.1016/j.isci.2020.101504
Activation of Cannabinoid Receptor 2 Prevents Colitis-Associated Colon Cancer through Myeloid Cell De-activation Upstream of IL-22 Production
Abstract
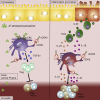
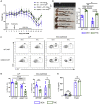
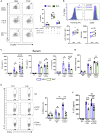
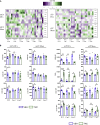
Intestinal disequilibrium leads to inflammatory bowel disease (IBD), and chronic inflammation predisposes to oncogenesis. Antigen-presenting dendritic cells (DCs) and macrophages can tip the equilibrium toward tolerance or pathology. Here we show that delta-9-tetrahydrocannabinol (THC) attenuates colitis-associated colon cancer and colitis induced by anti-CD40. Working through cannabinoid receptor 2 (CB2), THC increases CD103 expression on DCs and macrophages and upregulates TGF-β1 to increase T regulatory cells (Tregs). THC-induced Tregs are necessary to remedy systemic IFNγ and TNFα caused by anti-CD40, but CB2-mediated suppression of APCs by THC quenches pathogenic release of IL-22 and IL-17A in the colon. By examining tissues from multiple sites, we confirmed that THC affects DCs, especially in mucosal barrier sites in the colon and lungs, to reduce DC CD86. Using models of colitis and systemic inflammation we show that THC, through CB2, is a potent suppressor of aberrant immune responses by provoking coordination between APCs and Tregs.
Keywords: Cancer; Immunology.
© 2020 The Authors.
Conflict of interest statement
The authors have declared that no conflicts of interest exist.
Figures




References
-
- Brand S., Beigel F., Olszak T., Zitzmann K., Eichhorst S.T., Otte J.M., Diepolder H., Marquardt A., Jagla W., Popp A. IL-22 is increased in active Crohn’s disease and promotes proinflammatory gene expression and intestinal epithelial cell migration. Am. J. Physiol. Gastrointest. Liver Physiol. 2006;290:G827–G838. - PubMed
-
- Bauché D., Joyce-Shaikh B., Jain R., Grein J., Ku K.S., Blumenschein W.M., Ganal-Vonarburg S.C., Wilson D.C., McClanahan T.K., Malefyt R.d.W. LAG3+ regulatory T cells restrain interleukin-23-producing CX3CR1+ gut-resident macrophages during group 3 innate lymphoid cell-driven colitis. Immunity. 2018;49:342–352.e5. - PubMed
-
- Coward S., Clement F., Benchimol E.I., Bernstein C.N., Avina-Zubieta J.A., Bitton A., Carroll M.W., Hazlewood G., Jacobson K., Jelinski S. Past and future burden of inflammatory bowel diseases based on modeling of population-based data. Gastroenterology. 2019;156:1345–1353.e4. - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

