Resting-state functional connectivity and amyloid burden influence longitudinal cortical thinning in the default mode network in preclinical Alzheimer's disease
- PMID: 32942175
- PMCID: PMC7498941
- DOI: 10.1016/j.nicl.2020.102407
Resting-state functional connectivity and amyloid burden influence longitudinal cortical thinning in the default mode network in preclinical Alzheimer's disease
Abstract
Proteinopathies are key elements in the pathogenesis of age-related neurodegenerative diseases, particularly Alzheimer's disease (AD), with the nature and location of the proteinopathy characterizing much of the disease phenotype. Susceptibility of brain regions to pathology may partly be determined by intrinsic network structure and connectivity. It remains unknown, however, how these networks inform the disease cascade in the context of AD biomarkers, such as beta-amyloid (Aβ), in clinically-normal older adults.The default-mode network (DMN), a prominent intrinsic network, is heavily implicated in AD due to its spatial overlap with AD atrophy patterns and tau deposition. We investigated the influence of baseline Aβ positron emission tomography (PET) signal and intrinsic DMN connectivity on DMN-specific cortical thinning in 120 clinically-normal older adults from the Harvard Aging Brain Study (73 ± 6 years, 58% Female, CDR = 0). Participants underwent11C Pittsburgh Compound-B (PiB) PET, 18F flortaucipir (FTP) PET, and resting-state MRI scans at baselineand longitudinal MRI (3.6 ± 0.96 scans; 5.04 ± 0.8 years). Linear mixed models tested relationships between baseline PiB and DMN connectivity on cortical thinning in a composite of DMN regions. Lower DMN connectivity was associated with faster cortical thinning, but only in those with elevated baseline PiB-PET signal. This relationship was network specific, in that the frontoparietal control network did not account for the observed association. Additionally, the relationship was independent of inferior temporal lobe FTP-PET signal. Our findings provide evidence that compromised DMN connectivity, in the context of preclinical AD, foreshadows neurodegeneration in DMN regions.
Keywords: Alzheimer’s disease; Amyloid; Default mode network; Functional connectivity.
Copyright © 2020 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Dr. Johnson has served as a paid consultant for Bayer, GE Healthcare, Janssen, Siemens Medical Solutions, Genzyme, Novartis, Biogen, Roche, AZTherapy, GEHC, Lundbeck, Genentech, Lilly/Avid, AC Immune, and Abbvie. DDr. Sperling has received grants from National Institute on Aging, Alzheimer’s Association, Eli Lilly and Co., Janssen Pharmaceuticals, and Eisai. She has consulted for AC Immune, Eisai, Janssen, Prothena, Roche, and Takeda. There are no other conflicts of interest to be reported.
Figures
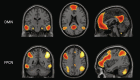


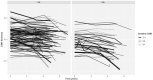
References
-
- Brier M.R., Gordon B., Friedrichsen K., McCarthy J., Stern A., Christensen J., Owen C., Aldea P., Su Y., Hassenstab J., Cairns N.J., Holtzman D.M., Fagan A.M., Morris J.C., Benzinger T.L.S., Ances B.M. Tau and AB imaging, CSF measures, and cognition in Alzheimer’s disease. Sci. Transl. Med. 2016;8(338):338RA66. - PMC - PubMed
-
- Buckner, R.L., Snyder, A.Z., Shannon, B.J., LaRossa, G., Sachs, R., Fotenos, A.F., Sheline, Y.I., Klunk, W.E., Mathis, C.A., Morris, J.C., Mintun, M.A., 2005. Molecular, structural, and functional characterization of Alzheimer’s disease: evidence for a relationship between default activity, amyloid, and memory. 25(34), 7709–7717. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

