Non-Human Primates Receiving High-Dose Total-Body Irradiation are at Risk of Developing Cerebrovascular Injury Years Postirradiation
- PMID: 32942304
- PMCID: PMC7583660
- DOI: 10.1667/RADE-20-00051.1
Non-Human Primates Receiving High-Dose Total-Body Irradiation are at Risk of Developing Cerebrovascular Injury Years Postirradiation
Abstract
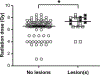
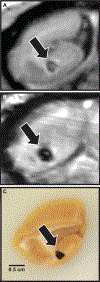
Nuclear accidents and acts of terrorism have the potential to expose thousands of people to high-dose total-body iradiation (TBI). Those who survive the acute radiation syndrome are at risk of developing chronic, degenerative radiation-induced injuries [delayed effects of acute radiation (DEARE)] that may negatively affect quality of life. A growing body of literature suggests that the brain may be vulnerable to radiation injury at survivable doses, yet the long-term consequences of high-dose TBI on the adult brain are unclear. Herein we report the occurrence of lesions consistent with cerebrovascular injury, detected by susceptibility-weighted magnetic resonance imaging (MRI), in a cohort of non-human primate [(NHP); rhesus macaque, Macaca mulatta] long-term survivors of high-dose TBI (1.1-8.5 Gy). Animals were monitored longitudinally with brain MRI (approximately once every three years). Susceptibility-weighted images (SWI) were reviewed for hypointensities (cerebral microbleeds and/or focal necrosis). SWI hypointensities were noted in 13% of irradiated NHP; lesions were not observed in control animals. A prior history of exposure was correlated with an increased risk of developing a lesion detectable by MRI (P = 0.003). Twelve of 16 animals had at least one brain lesion present at the time of the first MRI evaluation; a subset of animals (n = 7) developed new lesions during the surveillance period (3.7-11.3 years postirradiation). Lesions occurred with a predilection for white matter and the gray-white matter junction. The majority of animals with lesions had one to three SWI hypointensities, but some animals had multifocal disease (n = 2). Histopathologic evaluation of deceased animals within the cohort (n = 3) revealed malformation of the cerebral vasculature and remodeling of the blood vessel walls. There was no association between comorbid diabetes mellitus or hypertension with SWI lesion status. These data suggest that long-term TBI survivors may be at risk of developing cerebrovascular injury years after irradiation.
©2020 by Radiation Research Society. All rights of reproduction in any form reserved.
Figures


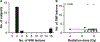

References
-
- Institute of Medicine (US) Committee on Medical Preparedness for a Terrorist Nuclear Event. Assessing medical preparedness to respond to a terrorist nuclear event: Workshop report. Benjamin GC, McGeary M, McCutchen SR, editors. Washington, DC: National Academies Press; 2009. - PubMed
-
- Fliedner TM, Dorr HD, Meineke V. Multi-organ involvement as a pathogenetic principle of the radiation syndromes: a study involving 110 case histories documented in SEARCH and classified as the bases of haematopoietic indicators of effect. Br J Radiol 2005; S27:1–8.
-
- Acute radiation syndrome: A brochure for physicians. Atlanta, GA: Centers for Disease Control and Prevention; 2017.
-
- Loganovsky K Do low doses of ionizing radiation affect the human brain? Data Sci J 2009; 8:PBR13–BR35.
-
- Danilov VM, Pozdeev VK. The epileptiform reactions of the human brain to prolonged exposure to low-dose ionizing radiation. (Article in Russian). Fiziol Zh Im I M Sechenova 1994; 80:88–98. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

