Soluble Cyanobacterial Carotenoprotein as a Robust Antioxidant Nanocarrier and Delivery Module
- PMID: 32942578
- PMCID: PMC7555398
- DOI: 10.3390/antiox9090869
Soluble Cyanobacterial Carotenoprotein as a Robust Antioxidant Nanocarrier and Delivery Module
Abstract
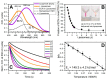
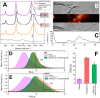
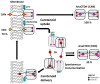
To counteract oxidative stress, antioxidants including carotenoids are highly promising, yet their exploitation is drastically limited by the poor bioavailability and fast photodestruction, whereas current delivery systems are far from being efficient. Here we demonstrate that the recently discovered nanometer-sized water-soluble carotenoprotein from Anabaena sp. PCC 7120 (termed AnaCTDH) transiently interacts with liposomes to efficiently extract carotenoids via carotenoid-mediated homodimerization, yielding violet-purple protein samples. We characterize the spectroscopic properties of the obtained pigment-protein complexes and the thermodynamics of liposome-protein carotenoid transfer and demonstrate the delivery of carotenoid echinenone from AnaCTDH into liposomes with an efficiency of up to 70 ± 3%. Most importantly, we show efficient carotenoid delivery to membranes of mammalian cells, which provides protection from reactive oxygen species (ROS). Incubation of neuroblastoma cell line Tet21N in the presence of 1 μM AnaCTDH binding echinenone decreased antimycin A ROS production by 25% (p < 0.05). The described carotenoprotein may be considered as part of modular systems for the targeted antioxidant delivery.
Keywords: carotenoid; carotenoid delivery; liposomes; oxidative stress; protein-membrane interactions; protein-protein interactions.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Konold P.E., van Stokkum I.H.M., Muzzopappa F., Wilson A., Groot M.L., Kirilovsky D., Kennis J.T.M. Photoactivation mechanism, timing of protein secondary structure dynamics and carotenoid translocation in the Orange Carotenoid Protein. J. Am. Chem. Soc. 2018;10:520–530. doi: 10.1021/jacs.8b11373. - DOI - PMC - PubMed
-
- Chew B.P., Park J.S., Wong M.W., Wong T.S. A comparison of the anticancer activities of dietary beta-carotene, canthaxanthin and astaxanthin in mice in vivo. Anticancer Res. 1999;19:1849–1853. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

