Glycoprotein G-protein Coupled Receptors in Disease: Luteinizing Hormone Receptors and Follicle Stimulating Hormone Receptors
- PMID: 32942611
- PMCID: PMC7565105
- DOI: 10.3390/diseases8030035
Glycoprotein G-protein Coupled Receptors in Disease: Luteinizing Hormone Receptors and Follicle Stimulating Hormone Receptors
Abstract
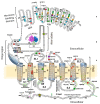
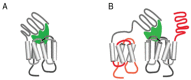
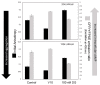
Signal transduction by luteinizing hormone receptors (LHRs) and follicle-stimulating hormone receptors (FSHRs) is essential for the successful reproduction of human beings. Both receptors and the thyroid-stimulating hormone receptor are members of a subset of G-protein coupled receptors (GPCRs) described as the glycoprotein hormone receptors. Their ligands, follicle-stimulating hormone (FSH) and luteinizing hormone (LH) and a structurally related hormone produced in pregnancy, human chorionic gonadotropin (hCG), are large protein hormones that are extensively glycosylated. Although the primary physiologic functions of these receptors are in ovarian function and maintenance of pregnancy in human females and spermatogenesis in males, there are reports of LHRs or FSHRs involvement in disease processes both in the reproductive system and elsewhere. In this review, we evaluate the aggregation state of the structure of actively signaling LHRs or FSHRs, their functions in reproduction as well as summarizing disease processes related to receptor mutations affecting receptor function or expression in reproductive and non-reproductive tissues. We will also present novel strategies for either increasing or reducing the activity of LHRs signaling. Such approaches to modify signaling by glycoprotein receptors may prove advantageous in treating diseases relating to LHRs or FSHRs function in addition to furthering the identification of new strategies for modulating GPCR signaling.
Keywords: follicle-stimulating hormone receptor; hormones; luteinizing hormone receptor.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Narayan P., Ulloa-Aguirre A., Dias J.A. Gonadotropin Hormones and Their Receptors. In: Strauss J.F., Barbieri R.L., editors. Yen and Jaffe’s Reproductive Endocrinology. 8th ed. Elsevier; Philadelphia, PA, USA: 2019. pp. 25–57.e15. Chapter 2.
-
- Casarini L., Huhtaniemi I., Simoni M., Rivero-Müller A. Gonadotrophin Receptors. Endocrinol. Testis Male Reprod. 2017;9:123–168.
-
- Wahlström T., Huhtaniemi I., Hovatta O., Seppälä M. Localization of luteinizing hormone, follicle-stimulating hormone, prolactin, and their receptors in human and rat testis using immunohistochemistry and radioreceptor assay. J. Clin. Endocrinol. Metab. 1983;57:825–830. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

