Bidirectional Ductal Shunting and Preductal to Postductal Oxygenation Gradient in Persistent Pulmonary Hypertension of the Newborn
- PMID: 32942726
- PMCID: PMC7552678
- DOI: 10.3390/children7090137
Bidirectional Ductal Shunting and Preductal to Postductal Oxygenation Gradient in Persistent Pulmonary Hypertension of the Newborn
Abstract
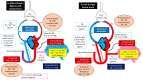
Background: The aim was to evaluate the relationship between the direction of the patent ductus arteriosus (PDA) shunt and the pre- and postductal gradient for arterial blood gas (ABG) parameters in a lamb model of meconium aspiration syndrome (MAS) with persistent pulmonary hypertension of the newborn (PPHN).
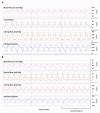
Methods: PPHN was induced by intermittent umbilical cord occlusion and the aspiration of meconium through the tracheal tube. After delivery, 13 lambs were ventilated and simultaneous 129 pairs of pre- and postductal ABG were drawn (right carotid and umbilical artery, respectively) while recording the PDA and the carotid and pulmonary blood flow.
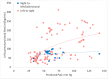
Results: Meconium aspiration resulted in hypoxemia. The bidirectional ductal shunt had a lower postductal partial arterial oxygen tension ([PaO2] with lower PaO2/FiO2 ratio-97 ± 36 vs. 130 ± 65 mmHg) and left pulmonary flow (81 ± 52 vs. 133 ± 82 mL/kg/min). However, 56% of the samples with a bidirectional shunt had a pre- and postductal saturation gradient of < 3%.
Conclusions: The presence of a bidirectional ductal shunt is associated with hypoxemia and low pulmonary blood flow. The absence of a pre- and postductal saturation difference is frequently observed with bidirectional right-to-left shunting through the PDA, and does not exclude a diagnosis of PPHN in this model.
Keywords: oxygenation saturation; patent ductus arteriosus; pulmonary hypertension.
Conflict of interest statement
The authors declare no conflict of interest.
Figures