Long-Term Advantages of Ovarian Reserve Maintenance and Follicle Development Using Adipose Tissue-Derived Stem Cells in Ovarian Tissue Transplantation
- PMID: 32942743
- PMCID: PMC7564479
- DOI: 10.3390/jcm9092980
Long-Term Advantages of Ovarian Reserve Maintenance and Follicle Development Using Adipose Tissue-Derived Stem Cells in Ovarian Tissue Transplantation
Abstract
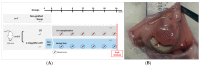
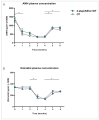
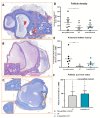
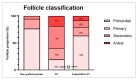
(1) Background: Ovarian tissue transplantation with adipose tissue-derived stem cells (ASCs) has been shown to enhance graft vascularization and increase follicle survival after a short interval of 7 days. The aim of the present study was to investigate their long-term effects on primordial follicle pool maintenance and follicle development. (2) Methods: A total of 14 severe combined immunodeficient (SCID) mice were grafted with frozen-thawed human ovarian tissue with or without ASCs. Blood was taken monthly in order to quantify the anti-Müllerian hormone (AMH) and estradiol. After 6 months, all the grafts were retrieved and sent for histology and immunolabeling (AMH, AMH receptor II, estrogen receptors α and β, and c-kit/kit ligand). (3) Results: A significant upturn was observed in AMH and estradiol plasma levels 4 months after transplantation in both grafted groups. The primordial follicle pool was better preserved in the ASC group (41.86 ± 28.35) than in the standard transplantation group (9.65 ± 17.6, p < 0.05) compared to non-grafted controls (124.7 ± 140). (4) Conclusions: The use of ASCs prior to ovarian tissue transplantation yielded a larger primordial follicle pool and more physiological follicle distribution after long-term grafting. These findings suggested that ASC use might extend the ovarian tissue lifespan.
Keywords: AMH; ASCs; adipose tissue-derived stem cells; endocrine restoration; estradiol; folliculogenesis; long-term transplantation; ovarian tissue transplantation.
Conflict of interest statement
None of the authors has any competing interests to declare in relation to this subject.
Figures

Similar articles
-
Two-step transplantation with adipose tissue-derived stem cells increases follicle survival by enhancing vascularization in xenografted frozen-thawed human ovarian tissue.Hum Reprod. 2018 Jun 1;33(6):1107-1116. doi: 10.1093/humrep/dey080. Hum Reprod. 2018. PMID: 29635371
-
Adipose tissue-derived stem cells protect the primordial follicle pool from both direct follicle death and abnormal activation after ovarian tissue transplantation.J Assist Reprod Genet. 2021 Jan;38(1):151-161. doi: 10.1007/s10815-020-02005-z. Epub 2020 Nov 12. J Assist Reprod Genet. 2021. PMID: 33184773 Free PMC article.
-
Effect of cryopreservation and transplantation on the expression of kit ligand and anti-Mullerian hormone in human ovarian tissue.Hum Reprod. 2012 Apr;27(4):1088-95. doi: 10.1093/humrep/des013. Epub 2012 Feb 7. Hum Reprod. 2012. PMID: 22313872
-
Translational research aiming to improve survival of ovarian tissue transplants using adipose tissue-derived stem cells.Acta Obstet Gynecol Scand. 2019 May;98(5):665-671. doi: 10.1111/aogs.13610. Epub 2019 Apr 13. Acta Obstet Gynecol Scand. 2019. PMID: 30891730 Review.
-
A putative role for anti-Müllerian hormone (AMH) in optimising ovarian reserve expenditure.J Endocrinol. 2017 Apr;233(1):R1-R13. doi: 10.1530/JOE-16-0522. Epub 2017 Jan 27. J Endocrinol. 2017. PMID: 28130407 Review.
Cited by
-
Effects of hypoxia-preconditioned HucMSCs on neovascularization and follicle survival in frozen/thawed human ovarian cortex transplanted to immunodeficient mice.Stem Cell Res Ther. 2022 Sep 14;13(1):474. doi: 10.1186/s13287-022-03167-6. Stem Cell Res Ther. 2022. PMID: 36104746 Free PMC article.
-
Restoring Ovarian Fertility and Hormone Function: Recent Advancements, Ongoing Efforts and Future Applications.J Endocr Soc. 2024 Apr 15;8(6):bvae073. doi: 10.1210/jendso/bvae073. eCollection 2024 Apr 6. J Endocr Soc. 2024. PMID: 38698870 Free PMC article. Review.
-
Research progress on mechanism of follicle injury after ovarian tissue transplantation and protective strategies.Zhejiang Da Xue Xue Bao Yi Xue Ban. 2024 Apr 1;53(3):321-330. doi: 10.3724/zdxbyxb-2023-0566. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2024. PMID: 38562041 Free PMC article. Review. Chinese, English.
-
Effects of needle puncturing on re-vascularization and follicle survival in xenotransplanted human ovarian tissue.Reprod Biol Endocrinol. 2023 Mar 20;21(1):28. doi: 10.1186/s12958-023-01081-x. Reprod Biol Endocrinol. 2023. PMID: 36941662 Free PMC article.
-
Design and Application Strategies of Natural Polymer Biomaterials in Artificial Ovaries.Ann Biomed Eng. 2023 Mar;51(3):461-478. doi: 10.1007/s10439-022-03125-6. Epub 2023 Jan 11. Ann Biomed Eng. 2023. PMID: 36629950 Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

