Effect of cholesterol re-supplementation and atorvastatin on plaque composition in the thoracic aorta of New Zealand white rabbits
- PMID: 32942987
- PMCID: PMC7499881
- DOI: 10.1186/s12872-020-01703-x
Effect of cholesterol re-supplementation and atorvastatin on plaque composition in the thoracic aorta of New Zealand white rabbits
Abstract
Background: Effects of re-supplementation of a cholesterol-enriched diet (CEDrs) on size, cholesterol content and morphology of already existing plaques are not known to date.
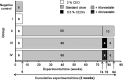
Methods: A group of rabbits received standard chow (SC) for 6 weeks ("negative control"; for plasma lipid measurements only). Group I-IV received 2% CED (induction) for 6 weeks; thereafter, groups II-IV have been fed a SC (= cholesterol withdrawal) for 68 weeks. Afterwards, feeding of groups II-IV was continued as follows: Group II - 10 weeks SC, group III - 4 weeks 0.5% CED (~re-supplementation), afterwards 6 weeks SC (~withdrawal again); group IV - 4 weeks 0.5% CED (re-supplementation) + atorvastatin (2.5 mg/kg body weight/day), afterwards 6 weeks SC (~withdrawal again) + atorvastatin. Plasma lipids, but also plaque size, morphology and cholesterol contents of thoracic aortas were quantified.
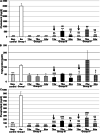
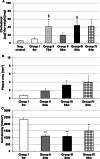
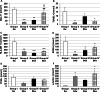
Results: After CEDrs, plasma cholesterol levels were increased. However, after withdrawal of CEDrs, plasma cholesterol levels decreased, whereas the cholesterol content of the thoracic aorta was increased in comparison with the group without CEDrs. Plaque size remained unaffected. Atorvastatin application did not change plasma cholesterol level, cholesterol content of the thoracic aorta and plaque size in comparison with the group without drug treatment. However, atorvastatin treatment increased the density of macrophages (MΦ) compared with the group without treatment, with a significant correlation between densities of MΦ (Mac-1+) and apoptotic (TUNEL+; TP53+), antigen-presenting (HLA-DR+) or oxidatively stressed (SOD2+) cells.
Conclusions: In rabbits with already existing plaques, CEDrs affects plaque morphology and cellular composition, but not plaque size. Despite missing effects on plasma cholesterol levels, cholesterol content of the thoracic aorta and size of already existing atherosclerotic plaques, atorvastatin treatment transforms the already existing lesions to a more active form, which may accelerate the remodelling to a more stable plaque.
Keywords: Apoptosis; Atherosclerosis; Atorvastatin; Hypercholesterolemic rabbits; Macrophage; Re-supplementation.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Cholesterol diet and effect of long-term withdrawal on plaque development and composition in the thoracic aorta of New Zealand White rabbits.Atherosclerosis. 2010 Jun;210(2):407-13. doi: 10.1016/j.atherosclerosis.2010.01.009. Epub 2010 Jan 22. Atherosclerosis. 2010. PMID: 20138623
-
Overexpression of Toll-Like Receptors 2, 3, 4, and 8 Is Correlated to the Vascular Atherosclerotic Process in the Hyperlipidemic Rabbit Model: The Effect of Statin Treatment.J Vasc Res. 2017;54(3):156-169. doi: 10.1159/000457797. Epub 2017 May 5. J Vasc Res. 2017. PMID: 28478461
-
Effect of lipid-lowering therapy with atorvastatin on atherosclerotic aortic plaques: a 2-year follow-up by noninvasive MRI.Eur J Cardiovasc Prev Rehabil. 2009 Apr;16(2):222-8. doi: 10.1097/HJR.0b013e32832948a0. Eur J Cardiovasc Prev Rehabil. 2009. PMID: 19242355 Clinical Trial.
-
Statins' Withdrawal Induces Atherosclerotic Plaque Destabilization in Animal Model-A "Rebound" Stimulation of Inflammation.J Cardiovasc Pharmacol Ther. 2019 Jul;24(4):377-386. doi: 10.1177/1074248419838499. Epub 2019 Mar 24. J Cardiovasc Pharmacol Ther. 2019. PMID: 30905179
-
Effect of atorvastatin on the expression of gamma-glutamyl transferase in aortic atherosclerotic plaques of apolipoprotein E-knockout mice.BMC Cardiovasc Disord. 2014 Oct 18;14:145. doi: 10.1186/1471-2261-14-145. BMC Cardiovasc Disord. 2014. PMID: 25326709 Free PMC article.
References
-
- Mach F, Baigent C, Catapano AL, Koskinas KC, Casul M, Badimon L, et al. 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk: the task force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and European atherosclerosis society (EAS) Eur Heart J. 2020;41:111–188. doi: 10.1093/eurheartj/ehz455. - DOI - PubMed
-
- Anitschkow N, Chalatow S. Ueber experimentelle cholesterinsteatose und ihre bedeutung für die entstehung einiger pathologischer prozesse. Zbl Allg Path path Anat. 1913;24:1–9.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

