NGF receptors and PI3K/AKT pathway involved in glucose fluctuation-induced damage to neurons and α-lipoic acid treatment
- PMID: 32943002
- PMCID: PMC7499848
- DOI: 10.1186/s12868-020-00588-y
NGF receptors and PI3K/AKT pathway involved in glucose fluctuation-induced damage to neurons and α-lipoic acid treatment
Abstract
Background: Glucose fluctuation promotes neuronal apoptosis, which plays a central role in diabetic encephalopathy (DE). Nerve growth factor (NGF), and its interaction with high-affinity (TrkA) and low-affinity (p75NTR) receptors, are involved in neuronal survival. NGF/TrkA contributes to the activation of the PI3K/AKT pathway, which is beneficial for neuronal survival, and α-Lipoic acid (ALA) exerts clinically favorable neuroprotective effects in the periphery. Whether NGF receptors and the PI3K/AKT pathway are involved in glucose fluctuation-induced neuronal damage, as well as the potential molecular mechanism of ALA in protecting glucose fluctuation-induced neuronal damage, remain unclear.
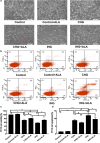
Results: The results indicated that constant high glucose (CHG) and intermittent high glucose (IHG) significantly increased the expression of Bax and caspase-3, and decreased the expression of TrkA/p75NTR and p-AKT/AKT, while ALA stimulation reversed the above proteins in PC12 cells. IHG stimulates apoptosis more effectively than CHG in PC12 cells, which is related to the PI3K/AKT pathway but not to the TrkA/p75NTR. Furthermore, neuronal apoptosis induced by IHG was aggravated by the TrkA inhibitor K252a or the PI3K/AKT inhibitor LY294002, but this effect was alleviated by the p75NTR inhibitor TAT-pep5.
Conclusion: Glucose fluctuation induced cell apoptosis by regulating the TrkA/p75NTR and PI3K/AKT pathway, meanwhile ALA exhibited neuroprotective effects in response to IHG and CHG. These observations indicated that the PI3K/AKT pathway and the balance of TrkA/p75NTR are likely to serve as potential therapeutic targets for DE. In addition, ALA could be a possible therapeutic drug for DE.
Keywords: Cell apoptosis; Diabetic encephalopathy; Intermittent high glucose; PI3K/AKT; TrkA; p75NTR.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures





References
-
- Barrett GL, Reid CA, Tsafoulis C, Zhu W, Williams DA, Paolini AG, Trieu J, Murphy M. Enhanced spatial memory and hippocampal long-term potentiation in p75 neurotrophin receptor knockout mice. Hippocampus. 2010;20(1):145–152. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

