Linc00514 promotes breast cancer metastasis and M2 polarization of tumor-associated macrophages via Jagged1-mediated notch signaling pathway
- PMID: 32943090
- PMCID: PMC7500027
- DOI: 10.1186/s13046-020-01676-x
Linc00514 promotes breast cancer metastasis and M2 polarization of tumor-associated macrophages via Jagged1-mediated notch signaling pathway
Abstract
Background: Tumor-associated macrophages (TAMs) and tumor cells are important components of the tumor microenvironment. M2 polarization of TAMs, which is a major actor in breast cancer malignancy and metastasis, can be induced by breast cancer cells. However, the potential mechanisms of the interaction between breast cancer cells and TAMs remain unclear.
Methods: The candidate breast cancer-associated long non-coding RNAs (lncRNAs) were analyzed using the GEO database. Functional assays, including MTT assay, Transwell assay, and EdU labeling detection, were performed to investigate the oncogenic role of linc00514 in breast cancer progression. The co-culture and ELISA assays were used to assess the role of linc00514 in macrophage recruitment and M2 polarization. RNA immunoprecipitation, RNA pull-down, and luciferase reporter assays were applied to determine the mechanism of linc00514 in breast cancer metastasis. Mouse xenograft models, mouse pulmonary metastatic models, and mouse primary tumor models were used to assess the role of linc00514 in M2 macrophage polarization and breast cancer tumorigenicity.
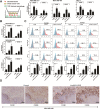
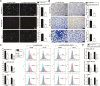
Results: Linc00514 was highly expressed in clinical breast cancer tissues and breast cancer cell lines. Overexpression of linc00514 promoted the proliferation and invasion of breast cancer cells and increased xenograft tumor volumes and pulmonary metastatic nodules. Overexpression of linc00514 also increased the percentage of macrophages expressing M2 markers CD206 and CD163. Mechanistically, linc00514 promoted Jagged1 expression in a transcriptional manner by increasing the phosphorylation of a transcription factor STAT3. Subsequently, Jagged1-mediated Notch signaling pathway promoted IL-4 and IL-6 secretions in breast cancer cells and ultimately inducing M2 polarization of macrophages.
Conclusion: Linc00514 plays an important role in regulating breast cancer tumorigenicity and M2 macrophage polarization via Jagged1-mediated Notch signaling pathway.
Keywords: Breast cancer; Jagged1; Tumor-associated macrophage; linc00514.
Conflict of interest statement
All authors declare that they have no competing interests.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

