Tocilizumab in treatment-naïve patients with Takayasu arteritis: TOCITAKA French prospective multicenter open-labeled trial
- PMID: 32943098
- PMCID: PMC7500024
- DOI: 10.1186/s13075-020-02311-y
Tocilizumab in treatment-naïve patients with Takayasu arteritis: TOCITAKA French prospective multicenter open-labeled trial
Abstract
Objectives: To assess long-term efficacy of tocilizumab in treatment-naive patients with Takayasu arteritis (TAK).
Methods: Prospective open-labeled trial in naïve patients with TAK who received steroids at the dose of 0.7 mg/kg/day and 7 infusions of 8 mg/kg/month of tocilizumab. The primary endpoint was the number of patients who discontinued steroids after 7 infusions of tocilizumab. Secondary endpoints included disease activity and the number of relapses during 18-month follow-up.
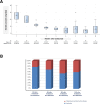
Results: Thirteen patients with TAK were included, with a median age of 32 years [19-45] and 12 (92%) females. Six (54%) patients met the primary end-point. A significant decrease of disease activity was observed after 6 months of tocilizumab therapy: decrease of median NIH scale (3 [3, 4] at baseline, versus 1 [0-2] after 6 months; p < 0.001), ITAS-2010 score (5 [2-7] versus 3 [0-8]; p = 0.002), and ITAS-A score (7 [4-10] versus 4 [1-15]; p = 0.0001)]. During the 12-month follow-up after tocilizumab discontinuation, a relapse occurred among 5 patients (45%) out of 11 in which achieved remission after 6 months of tocilizumab.
Conclusion: Tocilizumab seems an effective steroid sparing therapy in TAK, but maintenance therapy is necessary.
Trial registration: ClinicalTrials.gov NCT02101333 . Registered on 02 April 2014.
Keywords: Takayasu arteritis; Tocilizumab; Vasculitis treatment.
Conflict of interest statement
Coauthors declare no conflicts of interest for this study.
Figures
References
-
- Schmidt J, Kermani TA, Bacani AK, Crowson CS, Matteson EL, Warrington KJ. Tumor necrosis factor inhibitors in patients with Takayasu arteritis: experience from a referral center with long-term followup. Arthritis Care Res (Hoboken) 2012;64:1079–1083. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

