Four-octyl itaconate activates Nrf2 cascade to protect osteoblasts from hydrogen peroxide-induced oxidative injury
- PMID: 32943614
- PMCID: PMC7499214
- DOI: 10.1038/s41419-020-02987-9
Four-octyl itaconate activates Nrf2 cascade to protect osteoblasts from hydrogen peroxide-induced oxidative injury
Abstract
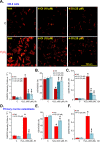
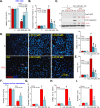
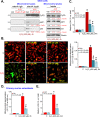
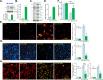
Four-octyl itaconate (4-OI) is the cell-permeable derivative of itaconate that can activate Nrf2 signaling by alkylating Keap1's cysteine residues. Here, we tested the potential effect of 4-OI on hydrogen peroxide (H2O2)-induced oxidative injury in osteoblasts. In OB-6 cells and primary murine osteoblasts, 4-OI was able to activate Nrf2 signaling cascade and cause Keap1-Nrf2 disassociation, Nrf2 protein stabilization, cytosol accumulation, and nuclear translocation. 4-OI also augmented antioxidant-response element reporter activity and promoted expression of Nrf2-dependent genes (HO1, NQO1, and GCLC). Pretreatment with 4-OI inhibited H2O2-induced reactive oxygen species production, cell death, and apoptosis in osteoblasts. Furthermore, 4-OI inhibited H2O2-induced programmed necrosis by suppressing mitochondrial depolarization, mitochondrial cyclophilin D-ANT1 (adenine nucleotide translocase 1)-p53 association, and cytosol lactate dehydrogenase release in osteoblasts. Ectopic overexpression of immunoresponsive gene 1 (IRG1) increased endogenous itaconate production and activated Nrf2 signaling cascade, thereby inhibiting H2O2-induced oxidative injury and cell death. In OB-6 cells, Nrf2 silencing or CRISPR/Cas9-induced Nrf2 knockout blocked 4-OI-induced osteoblast cytoprotection against H2O2. Conversely, forced Nrf2 activation, by CRISPR/Cas9-induced Keap1 knockout, mimicked 4-OI-induced actions in OB-6 cells. Importantly, 4-OI was ineffective against H2O2 in Keap1-knockout cells. Collectively, 4-OI efficiently activates Nrf2 signaling to inhibit H2O2-induced oxidative injury and death of osteoblasts.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

Similar articles
-
Four-octyl itaconate activates Keap1-Nrf2 signaling to protect neuronal cells from hydrogen peroxide.Cell Commun Signal. 2018 Nov 15;16(1):81. doi: 10.1186/s12964-018-0294-2. Cell Commun Signal. 2018. PMID: 30442144 Free PMC article.
-
A novel Keap1 inhibitor iKeap1 activates Nrf2 signaling and ameliorates hydrogen peroxide-induced oxidative injury and apoptosis in osteoblasts.Cell Death Dis. 2021 Jul 5;12(7):679. doi: 10.1038/s41419-021-03962-8. Cell Death Dis. 2021. PMID: 34226516 Free PMC article.
-
4-Octyl Itaconate Activates Nrf2 Signaling to Inhibit Pro-Inflammatory Cytokine Production in Peripheral Blood Mononuclear Cells of Systemic Lupus Erythematosus Patients.Cell Physiol Biochem. 2018;51(2):979-990. doi: 10.1159/000495400. Epub 2018 Nov 22. Cell Physiol Biochem. 2018. PMID: 30466076
-
Mechanisms underlying Nrf2 nuclear translocation by non-lethal levels of hydrogen peroxide: p38 MAPK-dependent neutral sphingomyelinase2 membrane trafficking and ceramide/PKCζ/CK2 signaling.Free Radic Biol Med. 2022 Oct;191:191-202. doi: 10.1016/j.freeradbiomed.2022.08.036. Epub 2022 Sep 3. Free Radic Biol Med. 2022. PMID: 36064071 Review.
-
Activation, interaction and intimation of Nrf2 pathway and their mutational studies causing Nrf2 associated cancer.Biochim Biophys Acta Mol Basis Dis. 2025 Jun;1871(5):167764. doi: 10.1016/j.bbadis.2025.167764. Epub 2025 Mar 14. Biochim Biophys Acta Mol Basis Dis. 2025. PMID: 40088576 Review.
Cited by
-
Nrf2 signaling activation by a small molecule activator compound 16 inhibits hydrogen peroxide-induced oxidative injury and death in osteoblasts.Cell Death Discov. 2022 Aug 8;8(1):353. doi: 10.1038/s41420-022-01146-7. Cell Death Discov. 2022. PMID: 35941127 Free PMC article.
-
Keap1-Nrf2 pathway: a key mechanism in the occurrence and development of cancer.Front Oncol. 2024 Apr 3;14:1381467. doi: 10.3389/fonc.2024.1381467. eCollection 2024. Front Oncol. 2024. PMID: 38634043 Free PMC article. Review.
-
4-Octyl Itaconate Prevents Free Fatty Acid-Induced Lipid Metabolism Disorder through Activating Nrf2-AMPK Signaling Pathway in Hepatocytes.Oxid Med Cell Longev. 2022 Feb 18;2022:5180242. doi: 10.1155/2022/5180242. eCollection 2022. Oxid Med Cell Longev. 2022. PMID: 35222799 Free PMC article.
-
Four-Octyl itaconate ameliorates periodontal destruction via Nrf2-dependent antioxidant system.Int J Oral Sci. 2022 May 31;14(1):27. doi: 10.1038/s41368-022-00177-1. Int J Oral Sci. 2022. PMID: 35637195 Free PMC article.
-
Negative regulation of pro-apoptotic AMPK/JNK pathway by itaconate in mice with fulminant liver injury.Cell Death Dis. 2023 Jul 31;14(7):486. doi: 10.1038/s41419-023-06001-w. Cell Death Dis. 2023. PMID: 37524706 Free PMC article.
References
-
- Schroder K. NADPH oxidases in bone homeostasis and osteoporosis. Free Radic. Biol. Med. 2019;132:67–72. - PubMed
-
- Ruan JW, Yao C, Bai JY, Zhou X. Z. microRNA-29a inhibition induces Gab1 upregulation to protect OB-6 human osteoblasts from hydrogen peroxide. Biochem. Biophys. Res. Commun. 2018;503:607–614. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

