Somatostatin 1.1 contributes to the innate exploration of zebrafish larva
- PMID: 32943676
- PMCID: PMC7499426
- DOI: 10.1038/s41598-020-72039-x
Somatostatin 1.1 contributes to the innate exploration of zebrafish larva
Abstract
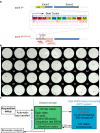
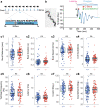
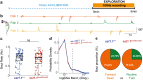
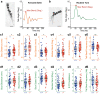
Pharmacological experiments indicate that neuropeptides can effectively tune neuronal activity and modulate locomotor output patterns. However, their functions in shaping innate locomotion often remain elusive. For example, somatostatin has been previously shown to induce locomotion when injected in the brain ventricles but to inhibit fictive locomotion when bath-applied in the spinal cord in vitro. Here, we investigated the role of somatostatin in innate locomotion through a genetic approach by knocking out somatostatin 1.1 (sst1.1) in zebrafish. We automated and carefully analyzed the kinematics of locomotion over a hundred of thousand bouts from hundreds of mutant and control sibling larvae. We found that the deletion of sst1.1 did not impact acousto-vestibular escape responses but led to abnormal exploration. sst1.1 mutant larvae swam over larger distance, at higher speed and performed larger tail bends, indicating that Somatostatin 1.1 inhibits spontaneous locomotion. Altogether our study demonstrates that Somatostatin 1.1 innately contributes to slowing down spontaneous locomotion.
Conflict of interest statement
O.M. work as a free-lance to develop the software ZebraZoom for other scientists in Academia (open-source software available for download, see
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

