Borophosphate glass as an active media for CuO nanoparticle growth: an efficient catalyst for selenylation of oxadiazoles and application in redox reactions
- PMID: 32943698
- PMCID: PMC7498614
- DOI: 10.1038/s41598-020-72129-w
Borophosphate glass as an active media for CuO nanoparticle growth: an efficient catalyst for selenylation of oxadiazoles and application in redox reactions
Abstract
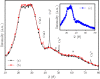
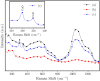
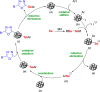
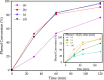
Herein, we report the preparation of CuO@ borophosphate nanoparticles (CuOnano@glass) and their wide catalytic applications. The glass annealing, under a controlled atmosphere, enables the growth of copper nanoparticles on the glass surface (not within) by an uncommon bottom-up process. Following the thermal annealing of metallic nanoparticles under air atmosphere, supported copper oxide nanoparticles CuONPs on the glass surface can be obtained. The approach enables the glass matrix to be explored as a precursor and a route for the synthesis of supported copper-based nanoparticles in a solvent-free process without immobilization steps or stabilizing agents. In order to demonstrate the wide synthetic utility of this CuONPs glass-based catalyst, one-pot three-component domino reactions were performed under an air atmosphere, affording the desired selenylated oxadiazoles in good to excellent yields. We also extended the application of these new materials as a glass-based catalyst in the phenol hydroxylation and the reduction of 4-nitrophenol.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Mauro JC, Zanotto ED. Two centuries of glass research: historical trends, current status, and grand challenges for the future. Int. J. Appl. Glass Sci. 2014;5:313–327. doi: 10.1111/ijag.12087. - DOI
-
- Belusso LC, et al. Synthesis of silver nanoparticles from bottom up approach on borophosphate glass and their applications as SERS, antibacterial and glass-based catalyst. Appl. Surf. Sci. 2019;473:303–312. doi: 10.1016/j.apsusc.2018.12.155. - DOI
-
- Chahine A, Et-tabirou M, Elbenaissi M, Haddad M, Pascal J. Effect of CuO on the structure and properties of (50–x/2)xCuO(50–x/2) glasses. Mater. Chem. Phys. 2004;84:341–347. doi: 10.1016/j.matchemphys.2003.11.009. - DOI
-
- Pereira AJ, et al. Facile shape-controlled fabrication of copper nanostructures on borophosphate glasses: synthesis, characterization, and their highly sensitive surface-enhanced raman scattering (SERS) properties. J. Phys. Chem. C. 2016;120:12265–12272. doi: 10.1021/acs.jpcc.6b02881. - DOI
Publication types
LinkOut - more resources
Full Text Sources

