Elevated acute phase proteins reflect peripheral inflammation and disease severity in patients with amyotrophic lateral sclerosis
- PMID: 32943739
- PMCID: PMC7499429
- DOI: 10.1038/s41598-020-72247-5
Elevated acute phase proteins reflect peripheral inflammation and disease severity in patients with amyotrophic lateral sclerosis
Abstract
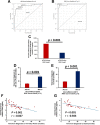
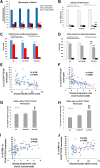
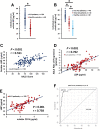
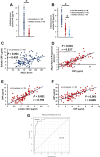
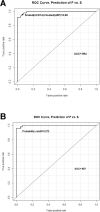
Amyotrophic lateral sclerosis (ALS) is a multifactorial, multisystem pro-inflammatory neuromuscular disorder compromising muscle function resulting in death. Neuroinflammation is known to accelerate disease progression and accentuate disease severity, but peripheral inflammatory processes are not well documented. Acute phase proteins (APPs), plasma proteins synthesized in the liver, are increased in response to inflammation. The objective of this study was to provide evidence for peripheral inflammation by examining levels of APPs, and their contribution to disease burden and progression rates. Levels of APPs, including soluble CD14 (sCD14), lipopolysaccharide binding protein (LBP), and C-reactive protein (CRP), were elevated in sera, and correlated positively with increased disease burden and faster progression. sCD14 was also elevated in patients' CSF and urine. After a 3 year follow-up, 72% of the patients with sCD14 levels above the receiver operating characteristics cutoff were deceased whereas only 28% below the cutoff were deceased. Furthermore, disease onset sites were associated with disease progression rates and APP levels. These APPs were not elevated in sera of patients with Alzheimer's Disease, frontotemporal dementia, or Parkinson's Disease. These collective APPs accurately reflect disease burden, progression rates, and survival times, reinforcing the concept of ALS as a disorder with extensive systemic pro-inflammatory responses.
Conflict of interest statement
DRB and SHA declare a conflict of interest with Implicit Bioscience. The other authors declare no competing interests.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

