Nociceptive mechanisms driving pain in a post-traumatic osteoarthritis mouse model
- PMID: 32943744
- PMCID: PMC7499425
- DOI: 10.1038/s41598-020-72227-9
Nociceptive mechanisms driving pain in a post-traumatic osteoarthritis mouse model
Abstract
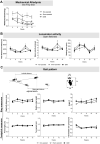
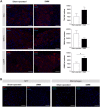
In osteoarthritis (OA), pain is the dominant clinical symptom, yet the therapeutic approaches remain inadequate. The knowledge of the nociceptive mechanisms in OA, which will allow to develop effective therapies for OA pain, is of utmost need. In this study, we investigated the nociceptive mechanisms involved in post-traumatic OA pain, using the destabilization of the medial meniscus (DMM) mouse model. Our results revealed the development of peripheral pain sensitization, reflected by augmented mechanical allodynia. Along with the development of pain behaviour, we observed an increase in the expression of calcitonin gene-related peptide (CGRP) in both the sensory nerve fibers of the periosteum and the dorsal root ganglia. Interestingly, we also observed that other nociceptive mechanisms commonly described in non-traumatic OA phenotypes, such as infiltration of the synovium by immune cells, neuropathic mechanisms and also central sensitization were not present. Overall, our results suggest that CGRP in the sensory nervous system is underlying the peripheral sensitization observed after traumatic knee injury in the DMM model, highlighting the CGRP as a putative therapeutic target to treat pain in post-traumatic OA. Moreover, our findings suggest that the nociceptive mechanisms involved in driving pain in post-traumatic OA are considerably different from those in non-traumatic OA.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

