New Insights into the Mechanism of Action of Viloxazine: Serotonin and Norepinephrine Modulating Properties
- PMID: 32943948
- PMCID: PMC7473988
- DOI: 10.2147/JEP.S256586
New Insights into the Mechanism of Action of Viloxazine: Serotonin and Norepinephrine Modulating Properties
Abstract
Background: Viloxazine was historically described as a norepinephrine reuptake inhibitor (NRI). Since NRIs have previously demonstrated efficacy in attention deficit/hyperactivity disorder (ADHD), viloxazine underwent contemporary investigation in the treatment of ADHD. Its clinical and safety profile, however, was found to be distinct from other ADHD medications targeting norepinephrine reuptake. Considering the complexity of neuropsychiatric disorders, understanding the mechanism of action (MoA) is an important differentiating point between viloxazine and other ADHD medications and provides pharmacology-based rationale for physicians prescribing appropriate therapy.
Methods: Viloxazine was evaluated in a series of in vitro binding and functional assays. Its effect on neurotransmitter levels in the brain was evaluated using microdialysis in freely moving rats.
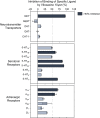
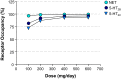
Results: We report the effects of viloxazine on serotoninergic (5-HT) system. In vitro, viloxazine demonstrated antagonistic activity at 5-HT2B and agonistic activity at 5-HT2C receptors, along with predicted high receptor occupancy at clinical doses. In vivo, viloxazine increased extracellular 5-HT levels in the prefrontal cortex (PFC), a brain area implicated in ADHD. Viloxazine also exhibited moderate inhibitory effects on the norepinephrine transporter (NET) in vitro and in vivo, and elicited moderate activity at noradrenergic and dopaminergic systems.
Conclusion: Viloxazine's ability to increase 5-HT levels in the PFC and its agonistic and antagonistic effects on certain 5-HT receptor subtypes, which were previously shown to suppress hyperlocomotion in animals, indicate that 5-HT modulating activity of viloxazine is an important (if not the predominant) component of its MoA, complemented by moderate NET inhibition. Supported by clinical data, these findings suggest the updated psychopharmacological profile of viloxazine can be best explained by its action as a serotonin norepinephrine modulating agent (SNMA).
Keywords: ADHD; SPN-812; mechanism of action; norepinephrine transporter; serotonin norepinephrine modulating agent; viloxazine.
© 2020 Yu et al.
Conflict of interest statement
CY, JGO, SC, and SS are employees of Supernus Pharmaceuticals, Inc. VM is an employee of the University of South Carolina School of Medicine. He is a consultant for ACADIA Pharmaceuticals Inc.; Alfasigma USA, Inc.; Alkermes, Inc.; Allergan; Eisai-Purdue; Intra-Cellular Therapies; Janssen; H. Lundbeck A/S; Otsuka America Pharmaceutical, Inc.; Sage Pharmaceuticals; Sunovion Pharmaceuticals Inc.; Supernus Pharmaceuticals, Inc.; and Takeda Pharmaceutical Company Limited. He serves on the speakers bureau of ACADIA Pharmaceuticals Inc.; Alkermes, Inc.; Allergan; Ironshore; Intra-Cellular; Janssen; H. Lundbeck A/S; Otsuka America Pharmaceutical, Inc.; Sunovion Pharmaceuticals Inc.; and Takeda Pharmaceutical Company Limited. The authors report no other conflicts of interest in this work.
Figures




References
-
- Foye WO. Pharmacodynamic agents In: Foye’s Principles of Medicinal Chemistry. Lemke TL, Williams DA, Roche VF, and Zito SW, eds. UK: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2013:610.
-
- Greenwood DT. International vivalan symposium; Animal pharmacology of viloxazine (Vivalan). J Int Med Res. 1975;3.
-
- Carlier PR, Lo MM, Lo PC, et al. Synthesis of a potent wide-spectrum serotonin-, norepinephrine-, dopamine-reuptake inhibitor (SNDRI) and a species-selective dopamine-reuptake inhibitor based on the gamma-amino alcohol functional group. Bioorg Med Chem Lett. 1998;8(5):487–492. doi: 10.1016/S0960-894X(98)00062-6. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

