Aryl Ether-Derived Sphingosine-1-Phosphate Receptor (S1P1) Modulators: Optimization of the PK, PD, and Safety Profiles
- PMID: 32944145
- PMCID: PMC7488280
- DOI: 10.1021/acsmedchemlett.0c00333
Aryl Ether-Derived Sphingosine-1-Phosphate Receptor (S1P1) Modulators: Optimization of the PK, PD, and Safety Profiles
Abstract
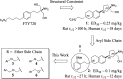
Efforts aimed at increasing the in vivo potency and reducing the elimination half-life of 1 and 2 led to the identification of aryl ether and thioether-derived bicyclic S1P1 differentiated modulators 3-6. The effects of analogs 3-6 on lymphocyte reduction in the rat (desired pharmacology) along with pulmonary- and cardiovascular-related effects (undesired pharmacology) are described. Optimization of the overall properties in the aryl ether series yielded 3d, and the predicted margin of safety against the cardiovascular effects of 3d would be large enough for human studies. Importantly, compared to 1 and 2, compound 3d had a better profile in both potency (ED50 < 0.05 mg/kg) and predicted human half-life (t 1/2 ∼ 5 days).
Copyright © 2020 American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



Similar articles
-
Bicyclic Ligand-Biased Agonists of S1P1: Exploring Side Chain Modifications to Modulate the PK, PD, and Safety Profiles.J Med Chem. 2021 Feb 11;64(3):1454-1480. doi: 10.1021/acs.jmedchem.0c01109. Epub 2021 Jan 25. J Med Chem. 2021. PMID: 33492963
-
Identification and Preclinical Pharmacology of ((1 R,3 S)-1-Amino-3-(( S)-6-(2-methoxyphenethyl)-5,6,7,8-tetrahydronaphthalen-2-yl)cyclopentyl)methanol (BMS-986166): A Differentiated Sphingosine-1-phosphate Receptor 1 (S1P1) Modulator Advanced into Clinical Trials.J Med Chem. 2019 Mar 14;62(5):2265-2285. doi: 10.1021/acs.jmedchem.8b01695. Epub 2019 Mar 6. J Med Chem. 2019. PMID: 30785748
-
Identification of potent tricyclic prodrug S1P1 receptor modulators.Medchemcomm. 2016 Nov 25;8(4):725-729. doi: 10.1039/c6md00539j. eCollection 2017 Apr 1. Medchemcomm. 2016. PMID: 30108791 Free PMC article.
-
Clinical pharmacology, efficacy, and safety aspects of sphingosine-1-phosphate receptor modulators.Expert Opin Drug Metab Toxicol. 2016 Aug;12(8):879-95. doi: 10.1080/17425255.2016.1196188. Epub 2016 Jun 13. Expert Opin Drug Metab Toxicol. 2016. PMID: 27249325 Review.
-
Modulators of Sphingosine-1-phosphate Pathway Biology: Recent Advances of Sphingosine-1-phosphate Receptor 1 (S1P1) Agonists and Future Perspectives.J Med Chem. 2017 Jul 13;60(13):5267-5289. doi: 10.1021/acs.jmedchem.6b01575. Epub 2017 Mar 30. J Med Chem. 2017. PMID: 28291340 Review.
Cited by
-
Multicomponent Synthesis of α-Branched Amines via a Zinc-Mediated Carbonyl Alkylative Amination Reaction.J Am Chem Soc. 2024 Apr 3;146(13):9045-9062. doi: 10.1021/jacs.3c14037. Epub 2024 Mar 15. J Am Chem Soc. 2024. PMID: 38488310 Free PMC article.
References
LinkOut - more resources
Full Text Sources

