Lactobacillus plantarum-derived extracellular vesicles induce anti-inflammatory M2 macrophage polarization in vitro
- PMID: 32944181
- PMCID: PMC7480564
- DOI: 10.1080/20013078.2020.1793514
Lactobacillus plantarum-derived extracellular vesicles induce anti-inflammatory M2 macrophage polarization in vitro
Abstract
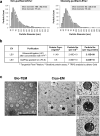
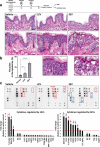
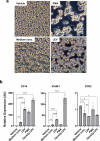
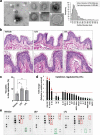
Probiotics offer various health benefits. Lactobacillus plantarum has been used for decades to enhance human intestinal mucosal immunity and improve skin barrier integrity. Extracellular vesicles (EVs) derived from eukaryotic or prokaryotic cells have been recognized as efficient carriers for delivery of biomolecules to recipient cells, and to efficiently regulate human pathophysiology. However, the mechanism underlying the beneficial effects of probiotic bacteria-derived EVs on human skin is unclear. Herein, we investigated how L. plantarum-derived EVs (LEVs) exert beneficial effects on human skin by examining the effect of LEVs on cutaneous immunity, particularly on macrophage polarization. LEVs promoted differentiation of human monocytic THP1 cells towards an anti-inflammatory M2 phenotype, especially M2b, by inducing biased expression of cell-surface markers and cytokines associated with M2 macrophages. Pre- or post-treatment with LEVs under inflammatory M1 macrophage-favouring conditions, induced by LPS and interferon-γ, inhibited M1-associated surface marker, HLA-DRα expression. Moreover, LEV treatment significantly induced expression of macrophage-characteristic cytokines, IL-1β, GM-CSF and the representative anti-inflammatory cytokine, IL-10, in human skin organ cultures. Hence, LEVs can trigger M2 macrophage polarization in vitro, and induce an anti-inflammatory phenomenon in the human skin, and may be a potent anti-inflammatory strategy to alleviate hyperinflammatory skin conditions.
Keywords: IL-10; Lactobacillus plantarum; Probiotics; extracellular vesicles; macrophage polarization.
© 2020 The Author(s). Published by Informa UK Limited, trading as Taylor & Francis Group on behalf of The International Society for Extracellular Vesicles.
Figures



References
-
- Toyofuku M, Nomura N, Eberl L.. Types and origins of bacterial membrane vesicles. Nat Rev Microbiol. 2019;17(1):13–24. - PubMed
-
- Kim JH, Lee J, Park J, et al. Gram-negative and Gram-positive bacterial extracellular vesicles. Semin Cell Dev Biol. 2015;40:97–104. - PubMed
-
- Choi E-J, Lee HG, Bae I-H, et al. Propionibacterium acnes-derived extracellular vesicles promote acne-like phenotypes in human epidermis. J Invest Dermatol. 2018;138(6):1371–1379. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

