Long-term efficacy and safety of eslicarbazepine acetate monotherapy for adults with newly diagnosed focal epilepsy: An open-label extension study
- PMID: 32944934
- PMCID: PMC7693183
- DOI: 10.1111/epi.16666
Long-term efficacy and safety of eslicarbazepine acetate monotherapy for adults with newly diagnosed focal epilepsy: An open-label extension study
Abstract
Objective: To assess the efficacy, safety, and tolerability of eslicarbazepine acetate (ESL) monotherapy during long-term treatment.
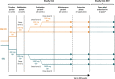
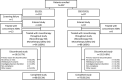
Methods: An open-label extension (OLE) study was conducted in adults completing a phase 3, randomized, double-blind, noninferiority trial, during which they had received monotherapy with either once-daily ESL or twice-daily controlled-release carbamazepine (CBZ-CR) for newly diagnosed focal epilepsy. In the OLE study, all patients received ESL (800-1600 mg/d) for 2 years. Primary efficacy outcome was retention time (from baseline of the OLE study). Secondary efficacy assessments included seizure freedom rate (no seizures during the OLE study) and responder rate (≥50% seizure frequency reduction from baseline of double-blind trial). Safety assessments included evaluation of treatment-emergent adverse events (TEAEs).
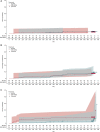
Results: Of 206 randomized patients, 96 who received ESL in the double-blind trial (ESL/ESL) and 88 who received CBZ-CR in the double-blind trial (CBZ-CR/ESL) were treated with ESL monotherapy (89.3% overall). Treatment retention time was similar between groups, with low probability of ESL withdrawal overall (<0.07 at any time). After 24 months, the probability of ESL withdrawal was 0.0638 (95% confidence interval [CI] = 0.0292-0.1366) in the ESL/ESL group and 0.0472 (95% CI = 0.0180-0.1210) in the CBZ-CR/ESL group. Seizure freedom rates were 90.6% (ESL/ESL) and 80.7% (CBZ-CR/ESL; P = .0531). Responder rates remained >80% in both groups throughout the study. Incidence of serious TEAEs was similar between groups (7.3% vs 5.7%; 0% vs 1.1% possibly related), as were the incidences of TEAEs considered at least possibly related to treatment (17.7% vs 18.2%) and TEAEs leading to discontinuation (3.1% vs 4.5%). The types of TEAEs were generally consistent with the known safety profile of ESL.
Significance: ESL monotherapy was efficacious and generally well tolerated over the long term, including in patients who transitioned from CBZ-CR monotherapy. No new safety concerns emerged.
Keywords: antiseizure medication; carbamazepine; focal seizures; responder rate; retention; seizure freedom rate.
© 2020 The Authors. Epilepsia published by Wiley Periodicals LLC on behalf of International League Against Epilepsy.
Conflict of interest statement
E.T. reports personal fees from EVER Pharma, Marinus, Argenix, Medtronic, Bial‐Portela & Cª, S.A., NewBridge, GL Pharma, GlaxoSmithKline, Boehringer Ingelheim, LivaNova, Eisai, UCB, Biogen, Genzyme Sanofi, and Actavis; his institution received grants from Biogen, UCB Pharma, Eisai, Red Bull, Merck, Bayer, the European Union, FWF Osterreichischer Fond zur Wissenschaftsforderung, Bundesministerium für Wissenschaft und Forschung, and Jubilaumsfond der Österreichischen Nationalbank outside the submitted work. R.R. is a consultant for Eisai, Bial, UCB, GlaxoSmithKline and Shire, and receives grant and research support from Bial, Eisai, and UCB. J.C. has received speaker's honoraria and/or consultancy fees from Bial and Eisai and was granted with a Tecnifar Bursary. J.M., F.I., and P.S.d.S. are current employees of Bial‐Portela & Cª, S.A. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Figures
References
-
- BIAL‐Portela & Cª . Zebinix®. Summary of product characteristics. 2019. Available at: https://www.ema.europa.eu/en/documents/product‐information/zebinix‐epar‐.... Accessed July 27, 2020.
-
- Sunovion Pharmaceuticals. Aptiom®. Highlights of prescribing information. 2019. Available at: http://www.aptiom.com/Aptiom‐Prescribing‐Information.pdf. Accessed July 27, 2020.
-
- Trinka E, Ben‐Menachem E, Kowacs PA, et al. Efficacy and safety of eslicarbazepine acetate versus controlled‐release carbamazepine monotherapy in newly diagnosed epilepsy: a phase III double‐blind, randomized, parallel‐group, multicenter study. Epilepsia. 2018;59(2):479–91. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

