Endoplasmic reticulum stress increases inflammatory cytokines in an epilepsy mouse model Gabrg2+/Q390X knockin: A link between genetic and acquired epilepsy?
- PMID: 32944937
- PMCID: PMC7918935
- DOI: 10.1111/epi.16670
Endoplasmic reticulum stress increases inflammatory cytokines in an epilepsy mouse model Gabrg2+/Q390X knockin: A link between genetic and acquired epilepsy?
Abstract
Objective: Neuroinflammation is a major theme in epilepsy, which has been characterized in acquired epilepsy but is poorly understood in genetic epilepsy. γ-Aminobutyric acid type A receptor subunit gene mutations are significant causes of epilepsy, and we have studied the pathophysiology directly resulting from defective receptor channels. Here, we determined the proinflammatory factors in a genetic mouse model, the Gabrg2+/Q390X knockin (KI). We have identified increased cytokines in multiple brain regions of the KI mouse throughout different developmental stages and propose that accumulation of the trafficking-deficient mutant protein may increase neuroinflammation, which would be a novel mechanism for genetic epilepsy.
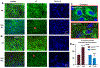
Methods: We used enzyme-linked immunosorbent assay, immunoprecipitation, nuclei purification, immunoblot, immunohistochemistry, and confocal microscopy to characterize increased neuroinflammation and its potential causes in a Gabrg2+/Q390X KI mouse and a Gabrg2+/- knockout (KO) mouse, each associated with a different epilepsy syndrome with different severities.
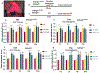
Results: We found that proinflammatory cytokines such as tumor necrosis factor alpha, interleukin 1-beta (IL-1β), and IL-6 were increased in the KI mice but not in the KO mice. A major underlying basis for the discrepancy in cytokine expression between the two mouse models is likely chronic mutant protein accumulation and endoplasmic reticulum (ER) stress. The presence of mutant protein dampened cytokine induction upon further cellular stimulation or external stress such as elevated temperature. Pharmacological induction of ER stress upregulated cytokine expression in the wild-type and KO but not in the KI mice. The increased cytokine expression was independent of seizure occurrence, because it was upregulated in both mice and cultured neurons.
Significance: Together, these data demonstrate a novel pathophysiology for genetic epilepsy, increased neuroinflammation, which is a common mechanism for acquired epilepsy. The findings thus provide the first link of neuroinflammation between genetic epilepsy associated with an ion channel gene mutation and acquired epilepsy.
Keywords: ER stress; GABAA receptors; Gabrg2+/Q390X knockin (KI) mice; epilepsy; neuroinflammation; proinflammatory cytokines.
© 2020 International League Against Epilepsy.
Conflict of interest statement
Figures

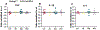


References
-
- Vezzani A, Balosso S, Ravizza T. Neuroinflammatory pathways as treatment targets and biomarkers in epilepsy. Nat Rev Neurol 2019;15:459–72. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

