Preictal variability of high-frequency oscillation rates in refractory epilepsy
- PMID: 32944942
- PMCID: PMC7722127
- DOI: 10.1111/epi.16680
Preictal variability of high-frequency oscillation rates in refractory epilepsy
Abstract
Objective: High-frequency oscillations (HFOs) have shown promising utility in the spatial localization of the seizure onset zone for patients with focal refractory epilepsy. Comparatively few studies have addressed potential temporal variations in HFOs, or their role in the preictal period. Here, we introduce a novel evaluation of the instantaneous HFO rate through interictal and peri-ictal epochs to assess their usefulness in identifying imminent seizure onset.
Methods: Utilizing an automated HFO detector, we analyzed intracranial electroencephalographic data from 30 patients with refractory epilepsy undergoing long-term presurgical evaluation. We evaluated HFO rates both as a 30-minute average and as a continuous function of time and used nonparametric statistical methods to compare individual and population-level differences in rate during peri-ictal and interictal periods.
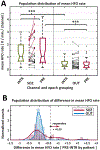
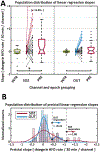
Results: Mean HFO rate was significantly higher for all epochs in seizure onset zone channels versus other channels. Across the 30 patients of our cohort, we found no statistically significant differences in mean HFO rate during preictal and interictal epochs. For continuous HFO rates in seizure onset zone channels, however, we found significant population-wide increases in preictal trends relative to interictal periods. Using a data-driven analysis, we identified a subset of 11 patients in whom either preictal HFO rates or their continuous trends were significantly increased relative to those of interictal baseline and the rest of the population.
Significance: These results corroborate existing findings that HFO rates within epileptic tissue are higher during interictal periods. We show this finding is also present in preictal, ictal, and postictal data, and identify a novel biomarker of preictal state: an upward trend in HFO rate leading into seizures in some patients. Overall, our findings provide preliminary evidence that HFOs can function as a temporal biomarker of seizure onset.
Keywords: high-frequency oscillation; preictal biomarker; seizure prediction; temporal biomarker.
© 2020 International League Against Epilepsy.
Conflict of interest statement
Disclosure
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
JS and SR have no conflicts of interest. WS and SG have a licensing agreement with Natus Medical, Inc but have received no financial remuneration.
Figures


References
-
- Cho JR, Koo DL, Joo EY, Seo DW, Hong SC, Jiruska P, et al. Resection of individually identified high-rate high-frequency oscillations region is associated with favorable outcome in neocortical epilepsy. Epilepsia. 2014; 55:1872–83. - PubMed

