Efficacy of early initiation of ivabradine treatment in patients with acute heart failure: rationale and design of SHIFT-AHF trial
- PMID: 32945150
- PMCID: PMC7754724
- DOI: 10.1002/ehf2.12997
Efficacy of early initiation of ivabradine treatment in patients with acute heart failure: rationale and design of SHIFT-AHF trial
Abstract
Aims: Elevated heart rate (HR) in heart failure (HF) is associated with worse outcomes, particularly in acute HF (AHF). HR reduction with ivabradine reduces cardiovascular events in HF patients with reduced ejection fraction. The present trial aimed to test the hypothesis that the early HR reduction using ivabradine improves clinical outcomes in patients with AHF.
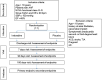
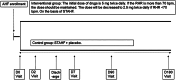
Methods and results: SHIFT-AHF is a prospective, multi-centre, double-blind, randomized, placebo-controlled trial to evaluate the efficacy and safety of ivabradine when adding to standard therapy in AHF patients (SHIFT-AHF). The trial will include 674 AHF patients with left ventricular ejection fraction < 45% and New York Heart Association functional classes III-IV. Participants were enrolled from March 2020 and will be followed up until December 2022. Patients are randomized to treatment with ivabradine or placebo (randomization 1:1). After allocation, the dose of ivabradine is titrated according to HR. Six months' follow-up and three control visits (7, 90, and 180 days after enrolment) are required for every participant. Assessment involves clinical examination, laboratory tests, echocardiography, electrocardiography, heart rhythm, cardiac function, and quality of life. The primary endpoint is a composite of all-cause mortality or re-admission due to worsening HF. Secondary endpoints include the assessments of cardiac remodelling, cardiac functional capacity, and quality of life.
Conclusions: The SHIFT-AHF trial will shed further light on the role of early HR reduction using ivabradine in patients with AHF.
Keywords: Acute heart failure; Ivabradine; Outcomes; Randomized controlled trial.
© 2020 The Authors. ESC Heart Failure published by John Wiley & Sons Ltd on behalf of the European Society of Cardiology.
Conflict of interest statement
None declared.
Figures
Comment in
-
Comment on: Efficacy of early initiation of ivabradine treatment in patients with acute heart failure: Rationale and design of SHIFT-AHF trial.ESC Heart Fail. 2021 Apr;8(2):1725-1726. doi: 10.1002/ehf2.13258. Epub 2021 Feb 27. ESC Heart Fail. 2021. PMID: 33638613 Free PMC article. No abstract available.
Similar articles
-
Effect of ivabradine in patients with heart failure with preserved ejection fraction: the EDIFY randomized placebo-controlled trial.Eur J Heart Fail. 2017 Nov;19(11):1495-1503. doi: 10.1002/ejhf.876. Epub 2017 Apr 30. Eur J Heart Fail. 2017. PMID: 28462519 Clinical Trial.
-
Impact of ivabradine on the cardiac function of chronic heart failure reduced ejection fraction: Meta-analysis of randomized controlled trials.Clin Cardiol. 2021 Apr;44(4):463-471. doi: 10.1002/clc.23581. Epub 2021 Feb 27. Clin Cardiol. 2021. PMID: 33638556 Free PMC article. Review.
-
[Efficacy and safety analysis of ivabradine hydrochloride treatment of Chinese patients with chronic heart failure: subgroup analysis of Chinese patients in the SHIFT study].Zhonghua Xin Xue Guan Bing Za Zhi. 2017 Mar 24;45(3):190-197. doi: 10.3760/cma.j.issn.0253-3758.2017.03.005. Zhonghua Xin Xue Guan Bing Za Zhi. 2017. PMID: 28316174 Clinical Trial. Chinese.
-
Beneficial effects of ivabradine in patients with heart failure, low ejection fraction, and heart rate above 77 b.p.m.ESC Heart Fail. 2019 Dec;6(6):1199-1207. doi: 10.1002/ehf2.12513. Epub 2019 Oct 8. ESC Heart Fail. 2019. PMID: 31591826 Free PMC article. Clinical Trial.
-
Ivabradine: in adults with chronic heart failure with reduced left ventricular ejection fraction.Am J Cardiovasc Drugs. 2012 Dec 1;12(6):415-26. doi: 10.2165/11209990-000000000-00000. Am J Cardiovasc Drugs. 2012. PMID: 23181944 Review.
Cited by
-
The association between ivabradine and adverse cardiovascular events in acute decompensated HFrEF patients.ESC Heart Fail. 2021 Oct;8(5):4199-4210. doi: 10.1002/ehf2.13536. Epub 2021 Jul 29. ESC Heart Fail. 2021. PMID: 34327853 Free PMC article.
-
Acute Heart Failure in an Almost-Centenarian Patient With Symptomatic Severe Aortic Stenosis Treated With Ivabradine.Cureus. 2022 Dec 2;14(12):e32142. doi: 10.7759/cureus.32142. eCollection 2022 Dec. Cureus. 2022. PMID: 36601174 Free PMC article.
-
Comment on: Efficacy of early initiation of ivabradine treatment in patients with acute heart failure: Rationale and design of SHIFT-AHF trial.ESC Heart Fail. 2021 Apr;8(2):1725-1726. doi: 10.1002/ehf2.13258. Epub 2021 Feb 27. ESC Heart Fail. 2021. PMID: 33638613 Free PMC article. No abstract available.
-
The role of ivabradine in doxorubicin-induced cardiotoxicity: exploring of underlying argument.Inflammopharmacology. 2022 Dec;30(6):2441-2446. doi: 10.1007/s10787-022-01082-z. Epub 2022 Oct 11. Inflammopharmacology. 2022. PMID: 36219320 Free PMC article.
-
Successful treatment with ivabradine in a β-blocker-refractory patient with acute decompensated heart failure with reduced ejection fraction.Clin Case Rep. 2023 Mar 4;11(3):e6890. doi: 10.1002/ccr3.6890. eCollection 2023 Mar. Clin Case Rep. 2023. PMID: 36879680 Free PMC article.
References
-
- Ambrosy AP, Fonarow GC, Butler J, Chioncel O, Greene SJ, Vaduganathan M, Nodari S, Lam CSP, Sato N, Shah AN, Gheorghiade M. The global health and economic burden of hospitalizations for heart failure: lessons learned from hospitalized heart failure registries. J Am Coll Cardiol 2014; 63: 1123–1133. - PubMed
-
- Braunwald E. The war against heart failure: the Lancet lecture. Lancet 2015; 385: 812–824. - PubMed
-
- Ponikowski P, Voors A, Anker S, Bueno H, Cleland J, Coats A, Falk V, Gonzalez‐Juanatey J, Harjola V, Jankowska E, Jessup M, Linde C, Nihoyannopoulos P, Parissis J, Pieske B, Riley J, Rosano G, Ruilope L, Ruschitzka F, Rutten F, van der Meer P. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2016. Aug; 18: 891–975. - PubMed
-
- Bohm M, Borer JS, Camm J, Ford I, Lloyd SM, Komajda M, Tavazzi L, Talajic M, Lainscak M, Reil JC, Ukena C, Swedberg K. Twenty‐four‐hour heart rate lowering with ivabradine in chronic heart failure: insights from the SHIFT Holter substudy. Eur J Heart Fail 2015; 17: 518–526. - PubMed
-
- Fox K, Ford I, Steg PG, Tendera M, Ferrari R, Investigators B. Ivabradine for patients with stable coronary artery disease and left‐ventricular systolic dysfunction (BEAUTIFUL): a randomised, double‐blind, placebo‐controlled trial. Lancet 2008; 372: 807–816. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

