Metformin promotes cell proliferation and osteogenesis under high glucose condition by regulating the ROS‑AKT‑mTOR axis
- PMID: 32945402
- PMCID: PMC7453594
- DOI: 10.3892/mmr.2020.11391
Metformin promotes cell proliferation and osteogenesis under high glucose condition by regulating the ROS‑AKT‑mTOR axis
Abstract
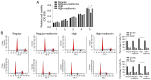
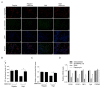
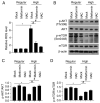
Metformin, a cost‑effective and safe orally administered antidiabetic drug used by millions of patients, has exhibited great interest for its potential osteogenic‑promoting properties in different types of cells, including mesenchymal stem cells (MSCs). Diabetic osteopathy is a common comorbidity of diabetes mellitus; however, the underlying molecular mechanisms of metformin on the physiological processes of MSCs, under high glucose condition, remain unknown. To determine the effects of metformin on the regulatory roles of proliferation and differentiation in MSCs, under high glucose conditions, osteogenesis after metformin treatment was detected with Alizarin Red S and ALP staining. The results demonstrated that high glucose levels significantly inhibited cell proliferation and osteogenic differentiation under high glucose conditions. Notably, addition of metformin reversed the inhibitory effects induced by high glucose levels on cell proliferation and osteogenesis. Furthermore, high glucose levels significantly decreased mitochondrial membrane potential (MMP), whereas treatment with metformin helped maintain MMP. Further analysis of mitochondrial function revealed that metformin significantly promoted ATP synthesis, mitochondrial DNA mass and mitochondrial transcriptional activity, which were inhibited by high glucose culture. Furthermore, metformin significantly scavenged reactive oxygen species (ROS) induced by high glucose levels, and regulated the ROS‑AKT‑mTOR axis inhibited by high glucose levels, suggesting the protective effects of metformin against high glucose levels via regulation of the ROS‑AKT‑mTOR axis. Taken together, the results of the present study demonstrated the protective role of metformin on the physiological processes of MSCs, under high glucose condition and highlighted the potential molecular mechanism underlying the effect of metformin in promoting cell proliferation and osteogenesis under high glucose condition.
Figures


Similar articles
-
Metformin-mediated effects on mesenchymal stem cells and mechanisms: proliferation, differentiation and aging.Front Pharmacol. 2024 Aug 13;15:1465697. doi: 10.3389/fphar.2024.1465697. eCollection 2024. Front Pharmacol. 2024. PMID: 39193338 Free PMC article. Review.
-
Metformin promotes differentiation of human bone marrow derived mesenchymal stem cells into osteoblast via GSK3β inhibition.Eur Rev Med Pharmacol Sci. 2018 Nov;22(22):7962-7968. doi: 10.26355/eurrev_201811_16424. Eur Rev Med Pharmacol Sci. 2018. PMID: 30536344
-
Metformin promotes osteogenic differentiation and protects against oxidative stress-induced damage in periodontal ligament stem cells via activation of the Akt/Nrf2 signaling pathway.Exp Cell Res. 2020 Jan 15;386(2):111717. doi: 10.1016/j.yexcr.2019.111717. Epub 2019 Nov 9. Exp Cell Res. 2020. PMID: 31715142
-
Improvement of osteogenic differentiation potential of placenta-derived mesenchymal stem cells by metformin via AMPK pathway activation.Stem Cell Res Ther. 2024 Nov 13;15(1):417. doi: 10.1186/s13287-024-04014-6. Stem Cell Res Ther. 2024. PMID: 39533406 Free PMC article.
-
Metformin enhances the osteogenesis and angiogenesis of human umbilical cord mesenchymal stem cells for tissue regeneration engineering.Int J Biochem Cell Biol. 2021 Dec;141:106086. doi: 10.1016/j.biocel.2021.106086. Epub 2021 Sep 20. Int J Biochem Cell Biol. 2021. PMID: 34551339
Cited by
-
Metformin Facilitates Osteoblastic Differentiation and M2 Macrophage Polarization by PI3K/AKT/mTOR Pathway in Human Umbilical Cord Mesenchymal Stem Cells.Stem Cells Int. 2022 Jun 18;2022:9498876. doi: 10.1155/2022/9498876. eCollection 2022. Stem Cells Int. 2022. PMID: 35761829 Free PMC article.
-
Osteoporosis in Adrenal Insufficiency: Could Metformin be Protective?Indian J Clin Biochem. 2025 Jan;40(1):4-11. doi: 10.1007/s12291-023-01153-0. Epub 2023 Sep 23. Indian J Clin Biochem. 2025. PMID: 39835225 Review.
-
The Function of Metformin in Aging-Related Musculoskeletal Disorders.Front Pharmacol. 2022 Mar 8;13:865524. doi: 10.3389/fphar.2022.865524. eCollection 2022. Front Pharmacol. 2022. PMID: 35392559 Free PMC article. Review.
-
Metformin Promotes Proliferation of Mouse Female Germline Stem Cells by Histone Acetylation Modification of Traf2.Stem Cell Rev Rep. 2023 Oct;19(7):2329-2340. doi: 10.1007/s12015-023-10575-5. Epub 2023 Jun 24. Stem Cell Rev Rep. 2023. PMID: 37354386
-
Metformin-mediated effects on mesenchymal stem cells and mechanisms: proliferation, differentiation and aging.Front Pharmacol. 2024 Aug 13;15:1465697. doi: 10.3389/fphar.2024.1465697. eCollection 2024. Front Pharmacol. 2024. PMID: 39193338 Free PMC article. Review.
References
-
- Harvey NC, McCloskey EV, Mitchell PJ, Dawson-Hughes B, Pierroz DD, Reginster JY, Rizzoli R, Cooper C, Kanis JA. Mind the (treatment) gap: A global perspective on current and future strategies for prevention of fragility fractures. Osteoporos Int. 2017;28:1507–1529. doi: 10.1007/s00198-016-3894-y. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

