AMD3100 and SDF‑1 regulate cellular functions of endothelial progenitor cells and accelerate endothelial regeneration in a rat carotid artery injury model
- PMID: 32945467
- PMCID: PMC7453604
- DOI: 10.3892/mmr.2020.11432
AMD3100 and SDF‑1 regulate cellular functions of endothelial progenitor cells and accelerate endothelial regeneration in a rat carotid artery injury model
Abstract
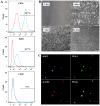
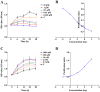
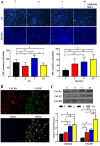
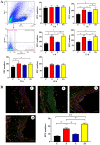
The present study was conducted to assess the effects of AMD3100 and stromal cell-derived factor 1 (SDF-1) on cellular functions and endothelial regeneration of endothelial progenitor cells (EPCs). The cell proliferation and adhesion capacity of EPCs were evaluated in vitro following treatment with AMD3100 and SDF‑1 using a Cell Counting Kit‑8 assay. Furthermore, the expression levels of C‑X‑C motif chemokine receptor 4 (CXCR4) and C‑X‑C motif chemokine receptor 7 (CXCR7) were detected before and after treatment with AMD3100 and SDF‑1 to elucidate their possible role in regulating the cellular function of EPCs. A rat carotid artery injury model was established to assess the influences of AMD3100 and SDF‑1 on endothelial regeneration. AMD3100 reduced the proliferation and adhesion capacity of EPCs to fibronectin (FN), whereas it increased the adhesion capacity of EPCs to human umbilical vein endothelial cells (HUVECs). However, SDF‑1 stimulated the proliferation and cell adhesion capacity of EPCs to HUVECs and FN. Additionally, the expression levels of CXCR7 but not CXCR4 were upregulated following AMD3100 treatment, whereas the expression levels of both CXCR4 and CXCR7 were upregulated after SDF‑1 treatment. In vivo results demonstrated that AMD3100 increased the number of EPCs in the peripheral blood and facilitated endothelial repair at 7 days after treatment. However, local administration of SDF‑1 alone did not enhance reendothelialization 7 and 14 days after treatment. Importantly, the combination of AMD3100 with SDF‑1 exhibited superior therapeutic effects compared with AMD3100 treatment alone, accelerated reendothelialization 7 days after treatment, and attenuated neointimal hyperplasia at day 7 and 14 by recruiting more EPCs to the injury site. In conclusion, AMD3100 could positively regulate the adhesion capacity of EPCs to HUVECs via elevation of the expression levels of CXCR7 but not CXCR4, whereas SDF‑1 could stimulate the proliferation and adhesion capacity of EPCs to FN and HUVECs by elevating the expression levels of CXCR4 and CXCR7. AMD3100 combined with SDF‑1 outperformed AMD3100 alone, promoted early reendothelialization and inhibited neointimal hyperplasia, indicating that early reendothelialization attenuated neointimal hypoplasia following endothelial injury.
Figures
Similar articles
-
Plerixafor stimulates adhesive activity and endothelial regeneration of endothelial progenitor cells via elevating CXCR7 expression.J Diabetes Complications. 2020 Oct;34(10):107654. doi: 10.1016/j.jdiacomp.2020.107654. Epub 2020 Jun 20. J Diabetes Complications. 2020. PMID: 32741660
-
The role of CXCR7 on the adhesion, proliferation and angiogenesis of endothelial progenitor cells.J Cell Mol Med. 2011 Jun;15(6):1299-309. doi: 10.1111/j.1582-4934.2011.01301.x. J Cell Mol Med. 2011. PMID: 21418513 Free PMC article.
-
Mobilization of endothelial progenitor cells promotes angiogenesis after full thickness excision by AMD3100 combined with G-CSF in diabetic mice by SDF-1/CXCR4 axis.Diab Vasc Dis Res. 2021 Mar-Apr;18(2):14791641211002473. doi: 10.1177/14791641211002473. Diab Vasc Dis Res. 2021. PMID: 33779350 Free PMC article.
-
Biological/pathological functions of the CXCL12/CXCR4/CXCR7 axes in the pathogenesis of bladder cancer.Int J Clin Oncol. 2017 Dec;22(6):991-1000. doi: 10.1007/s10147-017-1187-x. Epub 2017 Oct 11. Int J Clin Oncol. 2017. PMID: 29022185 Review.
-
CXCL12 (SDF1alpha)-CXCR4/CXCR7 pathway inhibition: an emerging sensitizer for anticancer therapies?Clin Cancer Res. 2011 Apr 15;17(8):2074-80. doi: 10.1158/1078-0432.CCR-10-2636. Epub 2011 Feb 24. Clin Cancer Res. 2011. PMID: 21349998 Free PMC article. Review.
Cited by
-
Atypical Roles of the Chemokine Receptor ACKR3/CXCR7 in Platelet Pathophysiology.Cells. 2022 Jan 9;11(2):213. doi: 10.3390/cells11020213. Cells. 2022. PMID: 35053329 Free PMC article. Review.
-
Outgrowth Endothelial Cell Conditioned Medium Negates TNF-α-Evoked Cerebral Barrier Damage: A Reverse Translational Research to Explore Mechanisms.Stem Cell Rev Rep. 2023 Feb;19(2):503-515. doi: 10.1007/s12015-022-10439-4. Epub 2022 Sep 2. Stem Cell Rev Rep. 2023. PMID: 36056287 Free PMC article.
-
Endothelial progenitor cells in the host defense response.Pharmacol Ther. 2023 Jan;241:108315. doi: 10.1016/j.pharmthera.2022.108315. Epub 2022 Nov 24. Pharmacol Ther. 2023. PMID: 36436689 Free PMC article. Review.
-
Inhibition of CXCR4 ameliorates hypoxia-induced pulmonary arterial hypertension in rats.Am J Transl Res. 2021 Mar 15;13(3):1458-1470. eCollection 2021. Am J Transl Res. 2021. PMID: 33841670 Free PMC article.
-
Impacts of systemic milieu on cerebrovascular and brain aging: insights from heterochronic parabiosis, blood exchange, and plasma transfer experiments.Geroscience. 2025 May 23. doi: 10.1007/s11357-025-01657-y. Online ahead of print. Geroscience. 2025. PMID: 40407975 Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

