Sinoporphyrin sodium is a promising sensitizer for photodynamic and sonodynamic therapy in glioma
- PMID: 32945475
- PMCID: PMC7448408
- DOI: 10.3892/or.2020.7695
Sinoporphyrin sodium is a promising sensitizer for photodynamic and sonodynamic therapy in glioma
Abstract
The aim of the present study was to explore the antitumor effects of sinoporphyrin sodium (DVDMS)‑mediated photodynamic therapy (PDT) and sonodynamic therapy (SDT) in glioma, and to reveal the underlying mechanisms. The uptake of DVDMS by U‑118 MG cells was detected by flow cytometry (FCM). A 630‑nm semiconductor laser and 1‑MHz ultrasound were used to perform PDT and SDT, respectively. Cell proliferation and apoptosis were evaluated using the Cell Counting Kit‑8 assay, FCM and Hoechst 33258 staining, respectively. Western blot analysis was used to detect protein expression and phosphorylation levels. BALB/c nude mice were used to establish a xenograft model of U‑118 MG cells. DVDMS was injected intravenously and PDT and SDT were performed 24 h later. An in vivo imaging system was used to evaluate the fluorescence of DVDMS, to measure tumor sizes, and to evaluate the therapeutic effects. The uptake of DVDMS by U‑118 MG cells was optimal after 4 h. PDT and SDT following DVDMS injection significantly inhibited the proliferation and increased apoptosis of glioma cells in vitro (P<0.05, P<0.01) respectively. In vivo, the fluorescence intensity of DVDMS was lower in the PDT and SDT groups compared with the DVDMS group, while tumor cell proliferation and weight were lower in the PDT and SDT groups than in the control group (P<0.05, P<0.01). However, there was no significant difference when laser, ultrasound or DVDMS were applied individually, compared with the control group. Hematoxylin and eosin staining suggested that both PDT and SDT induced significant apoptosis and vascular obstruction in cancer tissues. DVDMS‑mediated PDT and SDT inhibited the expression levels of proliferating cell nuclear antigen (PCNA) and Bcl‑xL, increased cleaved ‑caspase 3 levels, and decreased the protein phosphorylation of the PI3K/AKT/mTOR signaling pathway. Changes in the expression of PCNA, and Bcl‑xL and in the levels of cleaved‑caspase 3 were partly reversed by N‑acetyl‑L‑cysteine, a reactive oxygen species (ROS) scavenger. Similar results were obtained with FCM. DVDMS‑mediated PDT and SDT inhibited glioma cell proliferation and induced cell apoptosis in vitro and in vivo, potentially by increasing the generation of ROS and affecting protein expression and phosphorylation levels.
Keywords: sinoporphyrin sodium; photodynamic therapy; sonodynamic therapy; glioma; protein expression and phosphorylation.
Figures
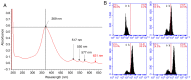

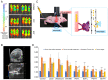

Similar articles
-
Sinoporphyrin Sodium-Mediated Sonodynamic Therapy Inhibits RIP3 Expression and Induces Apoptosis in the H446 Small Cell Lung Cancer Cell Line.Cell Physiol Biochem. 2018;51(6):2938-2954. doi: 10.1159/000496045. Epub 2018 Dec 14. Cell Physiol Biochem. 2018. PMID: 30562734
-
Sinoporphyrin sodium triggered sono-photodynamic effects on breast cancer both in vitro and in vivo.Ultrason Sonochem. 2016 Jul;31:437-48. doi: 10.1016/j.ultsonch.2016.01.038. Epub 2016 Jan 29. Ultrason Sonochem. 2016. PMID: 26964970
-
Sinoporphyrin sodium based sonodynamic therapy induces anti-tumor effects in hepatocellular carcinoma and activates p53/caspase 3 axis.Int J Biochem Cell Biol. 2019 Aug;113:104-114. doi: 10.1016/j.biocel.2019.01.009. Epub 2019 Jan 17. Int J Biochem Cell Biol. 2019. PMID: 30660690
-
The Application of DVDMS as a Sensitizing Agent for Sono-/Photo-Therapy.Front Pharmacol. 2020 Feb 7;11:19. doi: 10.3389/fphar.2020.00019. eCollection 2020. Front Pharmacol. 2020. PMID: 32116698 Free PMC article. Review.
-
Spotlight on porphyrins: Classifications, mechanisms and medical applications.Biomed Pharmacother. 2023 Aug;164:114933. doi: 10.1016/j.biopha.2023.114933. Epub 2023 May 24. Biomed Pharmacother. 2023. PMID: 37236030 Review.
Cited by
-
Applications of Focused Ultrasound for the Treatment of Glioblastoma: A New Frontier.Cancers (Basel). 2022 Oct 8;14(19):4920. doi: 10.3390/cancers14194920. Cancers (Basel). 2022. PMID: 36230843 Free PMC article. Review.
-
Sonodynamic Therapy for the Treatment of Intracranial Gliomas.J Clin Med. 2021 Mar 6;10(5):1101. doi: 10.3390/jcm10051101. J Clin Med. 2021. PMID: 33800821 Free PMC article. Review.
-
Research progress of berberine mediated photodynamic therapy.Oncol Lett. 2021 May;21(5):359. doi: 10.3892/ol.2021.12620. Epub 2021 Mar 8. Oncol Lett. 2021. PMID: 33747216 Free PMC article. Review.
-
Application of Self-Assembly Nanoparticles Based on DVDMS for Fenton-Like Ion Delivery and Enhanced Sonodynamic Therapy.Biosensors (Basel). 2022 Apr 18;12(4):255. doi: 10.3390/bios12040255. Biosensors (Basel). 2022. PMID: 35448315 Free PMC article.
-
Molecular Determinants for Photodynamic Therapy Resistance and Improved Photosensitizer Delivery in Glioma.Int J Mol Sci. 2024 Aug 9;25(16):8708. doi: 10.3390/ijms25168708. Int J Mol Sci. 2024. PMID: 39201395 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

