SUMOylation of HSP27 regulates PKM2 to promote esophageal squamous cell carcinoma progression
- PMID: 32945483
- PMCID: PMC7448476
- DOI: 10.3892/or.2020.7711
SUMOylation of HSP27 regulates PKM2 to promote esophageal squamous cell carcinoma progression
Abstract
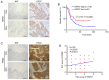
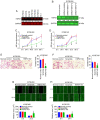
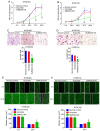
A previous proteomic screening of differentially expressed biomarkers between Kazakh patients with esophageal squamous cell carcinoma (ESCC) and normal adjacent tissues demonstrated that heat shock protein 27 (HSP27) and pyruvate kinase isoenzyme M2 (PKM2) were both highly expressed in ESCC samples compared with normal controls. However, the regulatory association between HSP27 and PKM2 in ESCC remains elusive. In the present study, immunohistochemistry and immunoblotting were adopted to examine the expression of HSP27, PKM2 and other relevant biomarkers involved in epithelial‑to‑mesenchymal transition in clinical tissue samples. The interactions between proteins were detected by co‑immunoprecipitation (Co‑IP) assay and further confirmed by immunofluorescence assay. The growth and motility of ESCC cells were examined by MTT, Transwell and wound healing assays. Overexpression of HSP27 was found to be significantly associated with T‑cell classification, lymph node metastasis and poor prognosis in ESCC. In addition, HSP27 expression was significantly correlated with PKM2 expression in ESCC specimens. Functionally, knockdown of HSP27 inhibited the growth and motility of ESCC cells. Moreover, HSP27 was found to directly interact with small ubiquitin‑related modified protein 2/3 (SUMO2/3) in ESCC cell lines, as evidenced by Co‑IP and laser confocal imaging. In addition, downregulation of HSP27 was shown to decrease PKM2 and E‑cadherin expression. Knockdown of SUMO2/3 was observed to reduce the expression of HSP27, PKM2 and EMT‑related biomarkers. The results of the present study indicated that the SUMOylation of HSP27 enhances the proliferation, invasion and migration of ESCC cells via PKM2.
Keywords: esophageal squamous cell carcinoma; heat shock protein 27; SUMOylation; pyruvate kinase isoenzyme M2.
Figures


Similar articles
-
M2 isoform of pyruvate kinase (PKM2) is upregulated in Kazakh's ESCC and promotes proliferation and migration of ESCC cells.Tumour Biol. 2016 Feb;37(2):2665-72. doi: 10.1007/s13277-015-4073-z. Epub 2015 Sep 24. Tumour Biol. 2016. PMID: 26404132
-
PSD3, regulated by PKM2, endows growth and metastasis advantages in esophageal squamous cell carcinoma by modulating EMT progression.Sci Rep. 2025 Jul 2;15(1):23212. doi: 10.1038/s41598-025-05649-y. Sci Rep. 2025. PMID: 40603347 Free PMC article.
-
Increased expression of HSP27 inhibits invasion and metastasis in human esophageal squamous cell carcinoma.Tumour Biol. 2014 Jul;35(7):6999-7007. doi: 10.1007/s13277-014-1946-5. Epub 2014 Apr 20. Tumour Biol. 2014. PMID: 24748206
-
Revisiting the Old Data of Heat Shock Protein 27 Expression in Squamous Cell Carcinoma: Enigmatic HSP27, More Than Heat Shock.Cells. 2022 May 17;11(10):1665. doi: 10.3390/cells11101665. Cells. 2022. PMID: 35626702 Free PMC article. Review.
-
Untangling the complexity of heat shock protein 27 in cancer and metastasis.Arch Biochem Biophys. 2023 Mar 1;736:109537. doi: 10.1016/j.abb.2023.109537. Epub 2023 Feb 3. Arch Biochem Biophys. 2023. PMID: 36738981 Review.
Cited by
-
Prognosis value of heat-shock proteins in esophageal and esophagogastric cancer: A systematic review and meta-analysis.World J Gastrointest Oncol. 2024 Apr 15;16(4):1578-1595. doi: 10.4251/wjgo.v16.i4.1578. World J Gastrointest Oncol. 2024. PMID: 38660660 Free PMC article.
-
Nanomedicine-driven tumor glucose metabolic reprogramming for enhanced cancer immunotherapy.Acta Pharm Sin B. 2025 Jun;15(6):2845-2866. doi: 10.1016/j.apsb.2025.04.002. Epub 2025 Apr 4. Acta Pharm Sin B. 2025. PMID: 40654336 Free PMC article. Review.
-
Suppressing SENP1 inhibits esophageal squamous carcinoma cell growth via SIRT6 SUMOylation.Cell Oncol (Dordr). 2025 Feb;48(1):67-81. doi: 10.1007/s13402-024-00956-4. Epub 2024 Jul 2. Cell Oncol (Dordr). 2025. PMID: 38954215 Free PMC article.
-
Immunoexpression of HSP27 does not seem to influence the prognosis of oral tongue squamous cell carcinoma.Braz Dent J. 2023 Dec 22;34(5):125-133. doi: 10.1590/0103-6440202305036. eCollection 2023. Braz Dent J. 2023. PMID: 38133467 Free PMC article.
-
Long Non-coding RNAs With In Vitro and In Vivo Efficacy in Preclinical Models of Esophageal Squamous Cell Carcinoma Which Act by a Non-microRNA Sponging Mechanism.Cancer Genomics Proteomics. 2022 Jul-Aug;19(4):372-389. doi: 10.21873/cgp.20327. Cancer Genomics Proteomics. 2022. PMID: 35732324 Free PMC article. Review.
References
-
- Liu Y, Liu J, Yin P, Liu S, Cai Y, You J, Zeng X, Wang L, Zhou M. The disease burden of malignant tumor in China, 1990 and 2010. Zhonghua Yu Fang Yi Xue Za Zhi. 2015;49:309–314. (In Chinese) - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

