Ibrutinib does not have clinically relevant interactions with oral contraceptives or substrates of CYP3A and CYP2B6
- PMID: 32945596
- PMCID: PMC7506988
- DOI: 10.1002/prp2.649
Ibrutinib does not have clinically relevant interactions with oral contraceptives or substrates of CYP3A and CYP2B6
Abstract
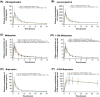
Ibrutinib may inhibit intestinal CYP3A4 and induce CYP2B6 and/or CYP3A. Secondary to potential induction, ibrutinib may reduce the exposure and effectiveness of oral contraceptives (OCs). This phase I study evaluated the effect of ibrutinib on the pharmacokinetics of the CYP2B6 substrate bupropion, CYP3A substrate midazolam, and OCs ethinylestradiol (EE) and levonorgestrel (LN). Female patients (N = 22) with B-cell malignancies received single doses of EE/LN (30/150 μg) and bupropion/midazolam (75/2 mg) during a pretreatment phase on days 1 and 3, respectively (before starting ibrutinib on day 8), and again after ibrutinib 560 mg/day for ≥ 2 weeks. Intestinal CYP3A inhibition was assessed on day 8 (single-dose ibrutinib plus single-dose midazolam). Systemic induction was assessed at steady-state on days 22 (EE/LN plus ibrutinib) and 24 (bupropion/midazolam plus ibrutinib). The geometric mean ratios (GMRs; test/reference) for maximum plasma concentration (Cmax ) and area under the plasma concentration-time curve (AUC) were derived using linear mixed-effects models (90% confidence interval within 80%-125% indicated no interaction). On day 8, the GMR for midazolam exposure with ibrutinib coadministration was ≤ 20% lower than the reference, indicating lack of intestinal CYP3A4 inhibition. At ibrutinib steady-state, the Cmax and AUC of EE were 33% higher than the reference, which was not considered clinically relevant. No substantial changes were noted for LN, midazolam, or bupropion. No unexpected safety findings were observed. A single dose of ibrutinib did not inhibit intestinal CYP3A4, and repeated administration did not induce CYP3A4/2B6, as assessed using EE, LN, midazolam, and bupropion.
Trial registration: ClinicalTrials.gov NCT03301207.
Keywords: Cytochrome P450; drug interactions; pharmacokinetics; phase I.
© 2020 The Authors. Pharmacology Research & Perspectives published by John Wiley & Sons Ltd, British Pharmacological Society and American Society for Pharmacology and Experimental Therapeutics.
Conflict of interest statement
The authors declare the following conflicts of interest: JdJ, AM, JJ, DO, and PH are/were employees of Janssen Research & Development, LLC, and hold stock in the company. WJ has served as a consultant or in an advisory role for Janssen‐Cilag, Acerta Pharma, Sandoz‐Novartis, Celltrion, MEI Pharma, Roche, and Gilead Sciences and has received research funding from Janssen‐Cilag, Acerta Pharma, Merck, Gilead Sciences, TG Therapeutics, Pfizer, Incyte, Bayer HealthCare Pharmaceuticals, Sandoz‐Novartis, Roche, Celltrion, Takeda Pharmaceuticals, Affimed Therapeutics, and Epizyme. RC has served on speakers’ bureaus and advisory boards for and has received travel funding from Janssen. CP has served as a consultant or advisor for Bristol Myers Squibb and Kyowa Kirin and has received travel funding from Roche Pharma. TW has served on advisory boards for Janssen‐Cilag, Roche, Celgene, and Amgen and received research funding from Roche. MD‐D has served on advisory boards for Servier, AbbVie, and Roche. JS is an employee of Pharmacyclics LLC, an AbbVie company, and holds stock in the company.
Figures

References
-
- IMBRUVICA® (ibrutinib) . [prescribing information]. Pharmacyclics LLC, Sunnyvale, CA; Janssen Biotech, Inc., Horsham, PA, USA; 2019.
-
- IMBRUVICA (ibrutinib) [summary of product characteristics] . Janssen‐Cilag International NV, Beerse, Belgium; 2019. https://www.ema.europa.eu/en/documents/product‐information/imbruvica‐epa...
-
- Scheers E, Leclercq L, de Jong J, et al. Absorption, metabolism, and excretion of oral 14C radiolabeled ibrutinib: an open‐label, phase I, single‐dose study in healthy men. Drug Metab Dispos. 2015;43(2):289‐297. - PubMed
-
- de Vries R, Huang M, Bode N, et al. Bioanalysis of ibrutinib and its active metabolite in human plasma: selectivity issue, impact assessment and resolution. Bioanalysis. 2015;7(20):2713‐2724. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

