Assessing the Perturbing Effects of Drugs on Lipid Bilayers Using Gramicidin Channel-Based In Silico and In Vitro Assays
- PMID: 32945672
- PMCID: PMC7586341
- DOI: 10.1021/acs.jmedchem.0c00958
Assessing the Perturbing Effects of Drugs on Lipid Bilayers Using Gramicidin Channel-Based In Silico and In Vitro Assays
Abstract
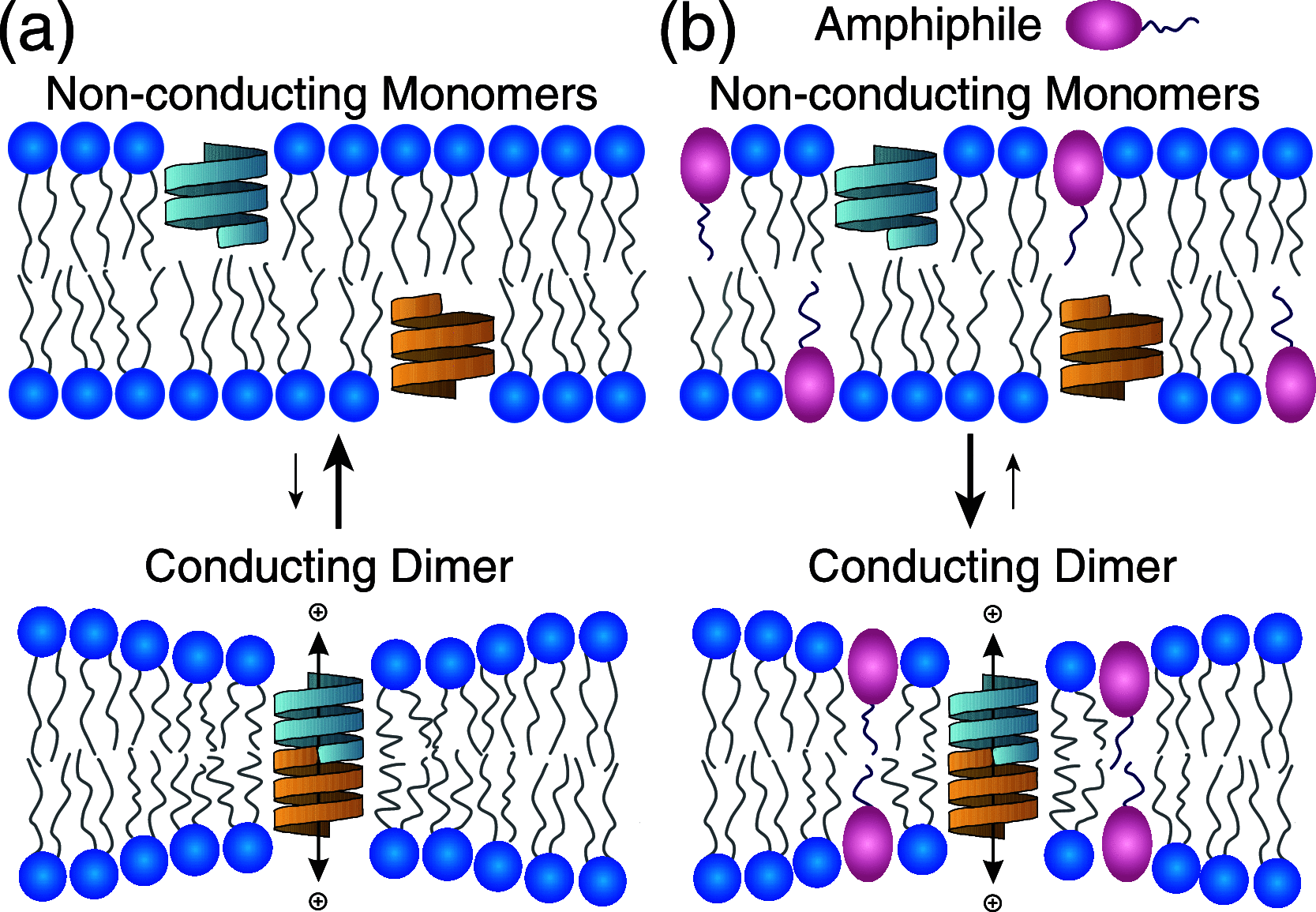
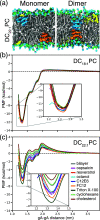
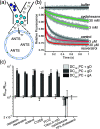
Partitioning of bioactive molecules, including drugs, into cell membranes may produce indiscriminate changes in membrane protein function. As a guide to safe drug development, it therefore becomes important to be able to predict the bilayer-perturbing potency of hydrophobic/amphiphilic drugs candidates. Toward this end, we exploited gramicidin channels as molecular force probes and developed in silico and in vitro assays to measure drugs' bilayer-modifying potency. We examined eight drug-like molecules that were found to enhance or suppress gramicidin channel function in a thick 1,2-dierucoyl-sn-glycero-3-phosphocholine (DC22:1PC) but not in thin 1,2-dioleoyl-sn-glycero-3-phosphocholine (DC18:1PC) lipid bilayer. The mechanism underlying this difference was attributable to the changes in gramicidin dimerization free energy by drug-induced perturbations of lipid bilayer physical properties and bilayer-gramicidin interactions. The combined in silico and in vitro approaches, which allow for predicting the perturbing effects of drug candidates on membrane protein function, have implications for preclinical drug safety assessment.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

Similar articles
-
Exchange of Gramicidin between Lipid Bilayers: Implications for the Mechanism of Channel Formation.Biophys J. 2017 Oct 17;113(8):1757-1767. doi: 10.1016/j.bpj.2017.08.049. Biophys J. 2017. PMID: 29045870 Free PMC article.
-
Gramicidin A Channel Formation Induces Local Lipid Redistribution I: Experiment and Simulation.Biophys J. 2017 Mar 28;112(6):1185-1197. doi: 10.1016/j.bpj.2017.01.028. Biophys J. 2017. PMID: 28355546 Free PMC article.
-
Molecular Mechanism for Gramicidin Dimerization and Dissociation in Bilayers of Different Thickness.Biophys J. 2019 Nov 19;117(10):1831-1844. doi: 10.1016/j.bpj.2019.09.044. Epub 2019 Oct 10. Biophys J. 2019. PMID: 31676135 Free PMC article.
-
Single-molecule methods for monitoring changes in bilayer elastic properties.Methods Mol Biol. 2007;400:543-70. doi: 10.1007/978-1-59745-519-0_37. Methods Mol Biol. 2007. PMID: 17951759 Review.
-
Lipid bilayer regulation of membrane protein function: gramicidin channels as molecular force probes.J R Soc Interface. 2010 Mar 6;7(44):373-95. doi: 10.1098/rsif.2009.0443. Epub 2009 Nov 25. J R Soc Interface. 2010. PMID: 19940001 Free PMC article. Review.
Cited by
-
Amphiphiles capsaicin and triton X-100 regulate the chemotherapy drug colchicine's membrane adsorption and ion pore formation potency.Saudi J Biol Sci. 2021 May;28(5):3100-3109. doi: 10.1016/j.sjbs.2021.02.054. Epub 2021 Feb 21. Saudi J Biol Sci. 2021. PMID: 34025185 Free PMC article.
-
Activity modulation of the Escherichia coli F1FO ATP synthase by a designed antimicrobial peptide via cardiolipin sequestering.iScience. 2023 May 30;26(7):107004. doi: 10.1016/j.isci.2023.107004. eCollection 2023 Jul 21. iScience. 2023. PMID: 37416464 Free PMC article.
-
Screening for bilayer-active and likely cytotoxic molecules reveals bilayer-mediated regulation of cell function.J Gen Physiol. 2023 Apr 3;155(4):e202213247. doi: 10.1085/jgp.202213247. Epub 2023 Feb 10. J Gen Physiol. 2023. PMID: 36763053 Free PMC article.
-
Peptide-induced membrane elastic deformations decelerate gramicidin dimer-monomer equilibration.Biophys J. 2021 Dec 7;120(23):5309-5321. doi: 10.1016/j.bpj.2021.10.030. Epub 2021 Oct 27. Biophys J. 2021. PMID: 34715080 Free PMC article.
-
Atomistic Characterization of Gramicidin Channel Formation.J Chem Theory Comput. 2021 Jan 12;17(1):7-12. doi: 10.1021/acs.jctc.0c00989. Epub 2020 Dec 30. J Chem Theory Comput. 2021. PMID: 33378617 Free PMC article.
References
-
- Ingólfsson H. I.; Thakur P.; Herold K. F.; Hobart E. A.; Ramsey N. B.; Periole X.; de Jong D. H.; Zwama M.; Yilmaz D.; Hall K.; Maretzky T.; Hemmings H. C. Jr.; Blobel C.; Marrink S. J.; Koçer A.; Sack J. T.; Andersen O. S. Phytochemicals Perturb Membranes and Promiscuously Alter Protein Function. ACS Chem. Biol. 2014, 9, 1788–1798. 10.1021/cb500086e. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

