Empagliflozin inhibits Na+ /H+ exchanger activity in human atrial cardiomyocytes
- PMID: 32946200
- PMCID: PMC7755005
- DOI: 10.1002/ehf2.13024
Empagliflozin inhibits Na+ /H+ exchanger activity in human atrial cardiomyocytes
Abstract
Aims: Recent clinical trials have proven gliflozins to be cardioprotective in diabetic and non-diabetic patients. However, the underlying mechanisms are incompletely understood. A potential inhibition of cardiac Na+ /H+ exchanger 1 (NHE1) has been suggested in animal models. We investigated the effect of empagliflozin on NHE1 activity in human atrial cardiomyocytes.
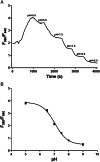
Methods and results: Expression of NHE1 was assessed in human atrial and ventricular tissue via western blotting. NHE activity was measured as the maximal slope of pH recovery after NH4 + pulse in isolated carboxy-seminaphtarhodafluor 1 (SNARF1)-acetoxymethylester-loaded murine ventricular and human atrial cardiomyocytes. NHE1 is abundantly expressed in human atrial and ventricular tissue. Interestingly, compared with patients without heart failure (HF), atrial NHE1 expression was significantly increased in patients with HF with preserved ejection fraction and atrial fibrillation. The largest increase in atrial and ventricular NHE1 expression, however, was observed in patients with end-stage HF undergoing heart transplantation. Importantly, acute exposure to empagliflozin (1 μmol/L, 10 min) significantly inhibited NHE activity to a similar extent in human atrial myocytes and mouse ventricular myocytes. This inhibition was also achieved by incubation with the well-described selective NHE inhibitor cariporide (10 μmol/L, 10 min).
Conclusions: This is the first study systematically analysing NHE1 expression in human atrial and ventricular myocardium of HF patients. We show that empagliflozin inhibits NHE in human cardiomyocytes. The extent of NHE inhibition was comparable with cariporide and may potentially contribute to the improved outcome of patients in clinical trials.
Keywords: Empagliflozin; Heart failure; Na+/H+ exchanger 1; SGLT2 inhibitor.
© 2020 The Authors. ESC Heart Failure published by John Wiley & Sons Ltd on behalf of the European Society of Cardiology.
Conflict of interest statement
M.A., L.S.M., and S.S. receive compensation for talks for Boehringer Ingelheim, the company that sells empagliflozin. The other authors declare to have no duality of interest associated with this manuscript.
Figures



References
-
- Grempler R, Thomas L, Eckhardt M, Himmelsbach F, Sauer A, Sharp DE, Bakker RA, Mark M, Klein T, Eickelmann P. Empagliflozin, a novel selective sodium glucose cotransporter‐2 (SGLT‐2) inhibitor: characterisation and comparison with other SGLT‐2 inhibitors. Diabetes Obes Metab 2012; 14: 83–90. - PubMed
-
- Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015; 373: 2117–2128. - PubMed
-
- McMurray JJV, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Anand IS, Bělohlávek J, Böhm M, Chiang C‐E, Chopra VK, de Boer RA, Desai AS, Diez M, Drozdz J, Dukát A, Ge J, Howlett JG, Katova T, Kitakaze M, Ljungman CEA, Merkely B, Nicolau JC, O'Meara E, Petrie MC, Vinh PN, Schou M, Tereshchenko S, Verma S, Held C, DeMets DL, Docherty KF, Jhund PS, Bengtsson O, Sjöstrand M, Langkilde A‐M. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med 2019; 381: 1995–2008. - PubMed
-
- Verma S, McMurray JJV. SGLT2 inhibitors and mechanisms of cardiovascular benefit: a state‐of‐the‐art review. Diabetologia 2018; 61: 2108–2117. - PubMed
-
- Mustroph J, Wagemann O, Lücht CM, Trum M, Hammer KP, Sag CM, Lebek S, Tarnowski D, Reinders J, Perbellini F, Terracciano C, Schmid C, Schopka S, Hilker M, Zausig Y, Pabel S, Sossalla ST, Schweda F, Maier LS, Wagner S. Empagliflozin reduces Ca/calmodulin‐dependent kinase II activity in isolated ventricular cardiomyocytes. ESC Heart Fail 2018; 5: 642–648. - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

