Identification, Structure-Activity Relationship, and Biological Characterization of 2,3,4,5-Tetrahydro-1 H-pyrido[4,3- b]indoles as a Novel Class of CFTR Potentiators
- PMID: 32946228
- PMCID: PMC8011931
- DOI: 10.1021/acs.jmedchem.0c01050
Identification, Structure-Activity Relationship, and Biological Characterization of 2,3,4,5-Tetrahydro-1 H-pyrido[4,3- b]indoles as a Novel Class of CFTR Potentiators
Abstract
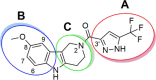
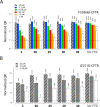
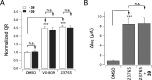
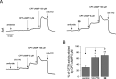
Cystic fibrosis (CF) is a life-threatening autosomal recessive disease, caused by mutations in the CF transmembrane conductance regulator (CFTR) chloride channel. CFTR modulators have been reported to address the basic defects associated with CF-causing mutations, partially restoring the CFTR function in terms of protein processing and/or channel gating. Small-molecule compounds, called potentiators, are known to ameliorate the gating defect. In this study, we describe the identification of the 2,3,4,5-tetrahydro-1H-pyrido[4,3-b]indole core as a novel chemotype of potentiators. In-depth structure-activity relationship studies led to the discovery of enantiomerically pure 39 endowed with a good efficacy in rescuing the gating defect of F508del- and G551D-CFTR and a promising in vitro druglike profile. The in vivo characterization of γ-carboline 39 showed considerable exposure levels and good oral bioavailability, with detectable distribution to the lungs after oral administration to rats. Overall, these findings may represent an encouraging starting point to further expand this chemical class, adding a new chemotype to the existing classes of CFTR potentiators.
Conflict of interest statement
The authors declare the following competing financial interest(s): T.B., F.B., F.G., S.G., F.S., E.C., L.F., N.P., and L.J.V.G. are inventors in a patent application protecting the class of compounds disclosed in this paper, and filed by Fondazione Istituto Italiano di Tecnologia, Istituto Giannina Gaslini, and Fondazione per la Ricerca sulla Fibrosi Cistica-Onlus (PCT international publication no. WO 2020/012427 A1; international publication date, 16 January 2020). The authors declare no other competing interests.
Figures








Similar articles
-
Mutation-specific potency and efficacy of cystic fibrosis transmembrane conductance regulator chloride channel potentiators.J Pharmacol Exp Ther. 2009 Sep;330(3):783-91. doi: 10.1124/jpet.109.154146. Epub 2009 Jun 2. J Pharmacol Exp Ther. 2009. PMID: 19491324 Free PMC article.
-
Current development of CFTR potentiators in the last decade.Eur J Med Chem. 2020 Oct 15;204:112631. doi: 10.1016/j.ejmech.2020.112631. Epub 2020 Jul 15. Eur J Med Chem. 2020. PMID: 32898816 Review.
-
Structure-activity relationship of 1,4-dihydropyridines as potentiators of the cystic fibrosis transmembrane conductance regulator chloride channel.Mol Pharmacol. 2007 Jul;72(1):197-207. doi: 10.1124/mol.107.034702. Epub 2007 Apr 23. Mol Pharmacol. 2007. PMID: 17452495
-
1-BENZYLSPIRO[PIPERIDINE-4,1'-PYRIDO[3,4-b]indole] 'co-potentiators' for minimal function CFTR mutants.Eur J Med Chem. 2021 Jan 1;209:112888. doi: 10.1016/j.ejmech.2020.112888. Epub 2020 Oct 8. Eur J Med Chem. 2021. PMID: 33092904 Free PMC article.
-
F508del-cystic fibrosis transmembrane regulator correctors for treatment of cystic fibrosis: a patent review.Expert Opin Ther Pat. 2015;25(9):991-1002. doi: 10.1517/13543776.2015.1045878. Epub 2015 May 15. Expert Opin Ther Pat. 2015. PMID: 25971311 Review.
Cited by
-
A Halogen-Atom Transfer (XAT)-Based Approach to Indole Synthesis Using Aryl Diazonium Salts and Alkyl Iodides.Org Lett. 2022 Nov 4;24(43):7883-7887. doi: 10.1021/acs.orglett.2c02840. Epub 2022 Oct 21. Org Lett. 2022. PMID: 36268790 Free PMC article.
-
Antischistosomal tetrahydro-γ-carboline sulfonamides.Bioorg Med Chem Lett. 2022 Mar 1;59:128546. doi: 10.1016/j.bmcl.2022.128546. Epub 2022 Jan 12. Bioorg Med Chem Lett. 2022. PMID: 35031451 Free PMC article.
-
Cystic Fibrosis Bone Disease: The Interplay between CFTR Dysfunction and Chronic Inflammation.Biomolecules. 2023 Feb 24;13(3):425. doi: 10.3390/biom13030425. Biomolecules. 2023. PMID: 36979360 Free PMC article. Review.
-
Understanding CFTR Functionality: A Comprehensive Review of Tests and Modulator Therapy in Cystic Fibrosis.Cell Biochem Biophys. 2024 Mar;82(1):15-34. doi: 10.1007/s12013-023-01200-w. Epub 2023 Dec 4. Cell Biochem Biophys. 2024. PMID: 38048024 Review.
-
Pharmacological Modulation of Ion Channels for the Treatment of Cystic Fibrosis.J Exp Pharmacol. 2021 Jul 23;13:693-723. doi: 10.2147/JEP.S255377. eCollection 2021. J Exp Pharmacol. 2021. PMID: 34326672 Free PMC article. Review.
References
-
- https://cftr2.org/mutations_history (accessed Mar 18, 2020).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information

