WDR90 is a centriolar microtubule wall protein important for centriole architecture integrity
- PMID: 32946374
- PMCID: PMC7500955
- DOI: 10.7554/eLife.57205
WDR90 is a centriolar microtubule wall protein important for centriole architecture integrity
Abstract
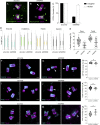
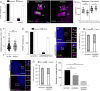
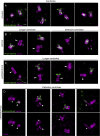
Centrioles are characterized by a nine-fold arrangement of microtubule triplets held together by an inner protein scaffold. These structurally robust organelles experience strenuous cellular processes such as cell division or ciliary beating while performing their function. However, the molecular mechanisms underlying the stability of microtubule triplets, as well as centriole architectural integrity remain poorly understood. Here, using ultrastructure expansion microscopy for nanoscale protein mapping, we reveal that POC16 and its human homolog WDR90 are components of the microtubule wall along the central core region of the centriole. We further found that WDR90 is an evolutionary microtubule associated protein. Finally, we demonstrate that WDR90 depletion impairs the localization of inner scaffold components, leading to centriole structural abnormalities in human cells. Altogether, this work highlights that WDR90 is an evolutionary conserved molecular player participating in centriole architecture integrity.
Keywords: cell biology; centriole; chlamydomonas; expansion microscopy; human; inner scaffold; microtubule.
Plain language summary
Cells are made up of compartments called organelles that perform specific roles. A cylindrical organelle called the centriole is important for a number of cellular processes, ranging from cell division to movement and signaling. Each centriole contains nine blades made up of protein filaments called microtubules, which link together to form a cylinder. This well-known structure can be found in a variety of different species. Yet, it is unclear how centrioles are able to maintain this stable architecture whilst carrying out their various different cell roles. In early 2020, a group of researchers discovered a scaffold protein at the center of centrioles that helps keep the microtubule blades stable. Further investigation suggested that another protein called WDR90 may also help centrioles sustain their cylindrical shape. However, the exact role of this protein was poorly understood. To determine the role of WDR90, Steib et al. – including many of the researchers involved in the 2020 study – used a method called Ultrastructure Expansion Microscopy to precisely locate the WDR90 protein in centrioles. This revealed that WDR90 is located on the microtubule wall of centrioles in green algae and human cells grown in the lab. Further experiments showed that the protein binds directly to microtubules and that removing WDR90 from human cells causes centrioles to lose their scaffold proteins and develop structural defects. This investigation provides fundamental insights into the structure and stability of centrioles. It shows that single proteins are key components in supporting the structural integrity of organelles and shaping their overall architecture. Furthermore, these findings demonstrate how ultrastructure expansion microscopy can be used to determine the role of individual proteins within a complex structure.
© 2020, Steib et al.
Conflict of interest statement
ES, ML, DG, NO, CZ, SB, VO, ML, FK, AT, MS, PG, VH No competing interests declared
Figures
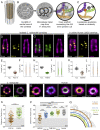

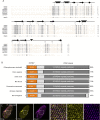
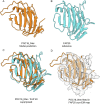
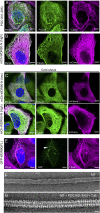
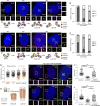
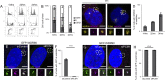
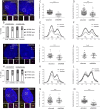
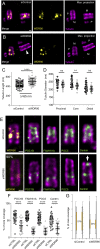



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

