Kinetics of Plasmodium midgut invasion in Anopheles mosquitoes
- PMID: 32946522
- PMCID: PMC7526910
- DOI: 10.1371/journal.ppat.1008739
Kinetics of Plasmodium midgut invasion in Anopheles mosquitoes
Abstract
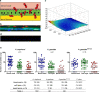
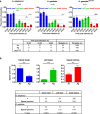
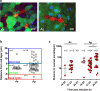
Malaria-causing Plasmodium parasites traverse the mosquito midgut cells to establish infection at the basal side of the midgut. This dynamic process is a determinant of mosquito vector competence, yet the kinetics of the parasite migration is not well understood. Here we used transgenic mosquitoes of two Anopheles species and a Plasmodium berghei fluorescence reporter line to track parasite passage through the mosquito tissues at high spatial resolution. We provide new quantitative insight into malaria parasite invasion in African and Indian Anopheles species and propose that the mosquito complement-like system contributes to the species-specific dynamics of Plasmodium invasion.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



Similar articles
-
The dynamics of interactions between Plasmodium and the mosquito: a study of the infectivity of Plasmodium berghei and Plasmodium gallinaceum, and their transmission by Anopheles stephensi, Anopheles gambiae and Aedes aegypti.Int J Parasitol. 2003 Aug;33(9):933-43. doi: 10.1016/s0020-7519(03)00112-7. Int J Parasitol. 2003. PMID: 12906877
-
Additional Feeding Reveals Differences in Immune Recognition and Growth of Plasmodium Parasites in the Mosquito Host.mSphere. 2021 Mar 31;6(2):e00136-21. doi: 10.1128/mSphere.00136-21. mSphere. 2021. PMID: 33789941 Free PMC article.
-
Immune resistance and tolerance strategies in malaria vector and non-vector mosquitoes.Parasit Vectors. 2017 Apr 18;10(1):186. doi: 10.1186/s13071-017-2109-5. Parasit Vectors. 2017. PMID: 28420446 Free PMC article.
-
Interactions of human malaria parasites, Plasmodium vivax and P.falciparum, with the midgut of Anopheles mosquitoes.Med Vet Entomol. 1997 Jul;11(3):290-6. doi: 10.1111/j.1365-2915.1997.tb00409.x. Med Vet Entomol. 1997. PMID: 9330262 Review.
-
Interactions between malaria parasites and their mosquito hosts in the midgut.Insect Biochem Mol Biol. 2004 Jul;34(7):679-85. doi: 10.1016/j.ibmb.2004.03.026. Insect Biochem Mol Biol. 2004. PMID: 15242709 Review.
Cited by
-
Divergent Plasmodium actin residues are essential for filament localization, mosquito salivary gland invasion and malaria transmission.PLoS Pathog. 2022 Aug 23;18(8):e1010779. doi: 10.1371/journal.ppat.1010779. eCollection 2022 Aug. PLoS Pathog. 2022. PMID: 35998188 Free PMC article.
-
Advances in the dissection of Anopheles-Plasmodium interactions.PLoS Pathog. 2025 Mar 31;21(3):e1012965. doi: 10.1371/journal.ppat.1012965. eCollection 2025 Mar. PLoS Pathog. 2025. PMID: 40163471 Free PMC article. Review.
-
Malaria parasites differentially sense environmental elasticity during transmission.EMBO Mol Med. 2021 Apr 9;13(4):e13933. doi: 10.15252/emmm.202113933. Epub 2021 Mar 5. EMBO Mol Med. 2021. PMID: 33666362 Free PMC article.
-
Driving down malaria transmission with engineered gene drives.Front Genet. 2022 Oct 19;13:891218. doi: 10.3389/fgene.2022.891218. eCollection 2022. Front Genet. 2022. PMID: 36338968 Free PMC article. Review.
-
Targeting the mosquito prefoldin-chaperonin complex blocks Plasmodium transmission.Nat Microbiol. 2025 Apr;10(4):841-854. doi: 10.1038/s41564-025-01947-3. Epub 2025 Mar 6. Nat Microbiol. 2025. PMID: 40050397
References
-
- WHO. WHO | World malaria report 2017. WHO. World Health Organization; 2018.
-
- Kiszewski A, Mellinger A, Spielman A, Malaney P, Sachs SE, Sachs J. A global index representing the stability of malaria transmission. Am J Trop Med Hyg. 2004;70(5):486–98. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

