SARS-CoV-2 identification and IgA antibodies in saliva: One sample two tests approach for diagnosis
- PMID: 32946791
- PMCID: PMC7492139
- DOI: 10.1016/j.cca.2020.09.018
SARS-CoV-2 identification and IgA antibodies in saliva: One sample two tests approach for diagnosis
Abstract
Aim: This study aims to verify whether standardized saliva collection is suitable for SARS-CoV-2 molecular detection and IgA measurement.
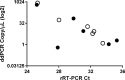
Methods: 43 COVID-19 inpatients and 326 screening subjects underwent naso-pharyngeal (NP)-swab and saliva collection (Salivette). Inpatients also underwent repeated blood collections to evaluate inflammation and organs involvement. In all patients and subjects, SARS-CoV-2 (gene E) rRT-PCR was undertaken in saliva and NP-swabs. Salivary IgA and serum IgA, IgG, IgM were measured on inpatients' samples.
Results: NP-swabs and saliva were both SARS-CoV-2 positive in 7 (16%) or both negative in 35 (82%) out of 43 patients successfully included in the study. NP-swabs and saliva results did not perfectly match in one patient (saliva positive, NP-swab negative). Positive molecular results were significantly associated with disease duration (p = 0.0049). 326/326 screening subjects were SARS-CoV-2 negative on both NP-swabs and saliva. Among the 27 saliva samples tested for IgA, 18 were IgA positive. Salivary IgA positivity was associated with pneumonia (p = 0.002) and CRP values (p = 0.0183), not with other clinical and molecular data, or with serum immunoglubulins.
Conclusions: A standardized saliva collection can be adopted to detect SARS-CoV-2 infection in alternative to NP-swabs. Preliminary data on salivary IgA support the use of saliva also for patient monitoring.
Keywords: Naso-pharyngeal swab; SARS-CoV-2; Saliva; Salivary IgA.
Copyright © 2020 Elsevier B.V. All rights reserved.
Figures

References
-
- Coronavirus disease 2019 (COVID-19) in the EU/EEA and the UK– ninth update, 2020, 1–50.
-
- C.B.E.M. Reusken, E.K. Broberg, B. Haagmans, A. Meijer, V.M. Corman, A. Papa, et al., Laboratory readiness and response for novel coronavirus (2019-nCoV) in expert laboratories in 30 EU/EEA countries, January 2020, Euro Surveill. 25 (6) (2020). http://doi.org/10.2807/1560-7917.ES.2020.25.6.2000082. - PMC - PubMed
-
- World Health Organization (WHO) Laboratory testing for 2019 novel coronavirus (2019-nCoV) in suspected human cases. https://apps.who.int/iris/handle/10665/330676, 2020 (accessed 15 June 2020).
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

