Neuropathic pain: clinical classification and assessment in patients with pain due to cancer
- PMID: 32947548
- PMCID: PMC7920493
- DOI: 10.1097/j.pain.0000000000002076
Neuropathic pain: clinical classification and assessment in patients with pain due to cancer
Abstract
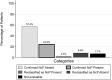
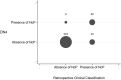
Neuropathic cancer pain (NcP) is associated with worse treatment responses and specific therapy indications, but a standardized clinical diagnosis of NcP is still lacking. This is a prospective observational study on outpatients with cancer, comparing different clinical approaches with NcP evaluation. A three-step assessment of NcP was performed using DN4 (cutoff of 4), palliative care physician Clinical Impression, including etiology and pain syndrome identification, and Retrospective Clinical Classification by a board of specialists with the IASP Neuropathic Pain Special Interest Group criteria. Neuropathic cancer pain classification was specifically referred to pain directly due to cancer. Three hundred fifty patients were assessed, and NcP prevalence was 20% (95% confidence interval [CI] 15.9%-24.6%), 36.9%, (95% CI 31.6%-42.1%), and 28.6% (95% CI 23.8%-33.9%) according to DN4, Clinical Impression, and Retrospective Clinical Classification, respectively. Cohen's kappa concordance coefficient between DN4 and Retrospective Clinical Classification was 0.57 (95% CI 0.47-0.67), indicating moderate concordance. Higher percentages of discordance were found for specific pain syndromes such as pain due to deep soft tissue infiltration and pain associated with tenesmus. Disagreement among clinicians accounted also for different NcP diagnoses and highlighted lack of homogeneous clinical criteria. Rigorous application of etiological and syndrome diagnosis to explain pain cause, associated with standardized diagnostic criteria and assessment of pain characteristics, that is also specific for the cancer pain condition could improve clinical classification of NcP.
Copyright © 2020 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the International Association for the Study of Pain.
Conflict of interest statement
A. Caraceni reports personal fees from Kyowa Kirin, Grunenthal GmbH, Pfizer, Almirall, Helsinn Healthcare, Molteni & C Soc Esercizio Spa, Shionogi, Italfarmaco, Sandoz International GmbH, and Institute de Recherche “Pierre Fabre” and grants from Molteni & C Soc Esercizio Spa, ProStrakan, Grunenthal GmbH, Amgen, and Ipsen, outside the submitted work. S. Kaasa reports personal fees from Fresenius Kabi, personal fees and grants from Nutricia, and other from Eir Solution, outside the submitted work. E. Zecca reports grants from Amgen srl, outside the submitted work. M. Shkodra reports grants from EU Research Framework Programme H2020/Marie Skłodowska-Curie Actions, during the conduct of the study, and other from Angelini, outside the submitted work. The remaining authors have no conflicts of interest to declare.
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Figures


References
-
- Bennett M. The LANSS Pain Scale: the Leeds assessment of neuropathic symptoms and signs. PAIN 2001;92:147–57. - PubMed
-
- Bennett MI, Kaasa S, Barke A, Korwisi B, Rief W, Treede RD; IASP Taskforce for the Classification of Chronic Pain. The IASP classification of chronic pain for ICD-11: chronic cancer-related pain. PAIN 2019;160:38–44. - PubMed
-
- Bennett MI, Rayment C, Hjermstad M, Aass N, Caraceni A, Kaasa S. Prevalence and aetiology of neuropathic pain in cancer patients: a systematic review. PAIN 2012;153:359–65. - PubMed
-
- Bennett MI. Gabapentin significantly improves analgesia in people receiving opioids for neuropathic cancer pain: abstracted from: Caraceni A, Zecca E, Bonezzi C, et al. Gabapentin for neuropathic cancer pain: a randomized controlled trial from the Gabapentin Cancer Pain Study Group. J Clin Oncol 2004; 22: 2909–17. Cancer Treat Rev 2005;31:58–62. - PubMed

