Methylation of Methyl 4-Hydroxy-2-thioxo-1,2-dihydroquinoline-3-carboxylate: Synthetic, Crystallographic, and Molecular Docking Studies
- PMID: 32947763
- PMCID: PMC7570464
- DOI: 10.3390/molecules25184238
Methylation of Methyl 4-Hydroxy-2-thioxo-1,2-dihydroquinoline-3-carboxylate: Synthetic, Crystallographic, and Molecular Docking Studies
Abstract
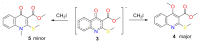
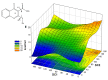
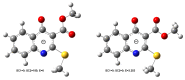
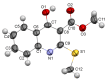
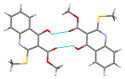
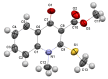
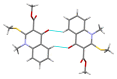
Consecutive alkylation of 4-hydroxy-2-thioxo-1,2-dihydroquinoline-3-carboxylate by CH3I has been investigated to establish regioselectivity of the reaction for reliable design and synthesis of combinatorial libraries. In the first stage, the product of S-methylation-methyl 4-hydroxy-2-(methylthio)quinoline-3-carboxylate was obtained. The subsequent alkylation with CH3I led to the formation of both O- and N-methylation products mixture-methyl 4-methoxy-2-(methylthio)quinoline-3-carboxylate and methyl 1-methyl-2-(methylthio)-4-oxo-1,4-dihydroquinoline-3-carboxylate with a predominance of O-methylated product. The structure of synthesized compounds was confirmed by means of elemental analysis, 1H-NMR, 13C-NMR, LC/MS, and single-crystal X-ray diffraction. The quantum chemical calculations of geometry and electron structure of methyl 4-hydroxy-2-(methylthio)quinoline-3-carboxylate's anion were carried out. According to molecular docking simulations, the studied compounds can be considered as potent inhibitors of Hepatitis B Virus replication. Experimental in vitro biological studies confirmed that studied compounds demonstrated high inhibition of HBV replication in 10 µM concentration.
Keywords: X-ray analysis; alkylation; hepatitis B virus; hydrogen bond; molecular docking simulations; quinoline.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








References
-
- Kazuno K., Kise M., Kitano M., Ozaki M., Segawa J., Shirahase I., Tomii Y. Preparation of Oxothiazetoquinolinecarboxylates as Bactericides. 2190376B. GB Patent. 1987 Nov 18;
-
- Taguchi M., Kondo H., Inoue Y., Kawahata Y., Tsukamoto G. Epoxymethanothiazoloquinolone carboxylic Acid Derivatives as Medical Bactericides, Their Preparation, and Formulations Containing Them. EPA 286089A1. 1988 Oct 12;
-
- Kise M., Kitano M., Ozaki M., Kazuno K., Matsuda M., Shirahase I., Segawa J. Preparation and Testing of 6-Fluoro-7-piperazino-4-oxo-4H-[1,3]thiazeto[3,2-a]quinolinecarboxylate Derivatives as Antibactericides. EPA 315828A1. 1989 May 17;
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

