HCV Glycoprotein Structure and Implications for B-Cell Vaccine Development
- PMID: 32947858
- PMCID: PMC7555785
- DOI: 10.3390/ijms21186781
HCV Glycoprotein Structure and Implications for B-Cell Vaccine Development
Abstract
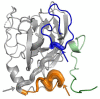
Despite the approval of highly efficient direct-acting antivirals in the last decade Hepatitis C virus (HCV) remains a global health burden and the development of a vaccine would constitute an important step towards the control of HCV. The high genetic variability of the viral glycoproteins E1 and E2, which carry the main neutralizing determinants, together with their intrinsic structural flexibility, the high level of glycosylation that shields conserved neutralization epitopes and immune evasion using decoy epitopes renders the design of an efficient vaccine challenging. Recent structural and functional analyses have highlighted the role of the CD81 receptor binding site on E2, which overlaps with those neutralization epitopes within E2 that have been structurally characterized to date. This CD81 binding site consists of three distinct segments including "epitope I", "epitope II" and the "CD81 binding loop". In this review we summarize the structural features of the HCV glycoproteins that have been derived from X-ray structures of neutralizing and non-neutralizing antibody fragments complexed with either recombinant E2 or epitope-derived linear peptides. We focus on the current understanding how neutralizing antibodies interact with their cognate antigen, the structural features of the respective neutralization epitopes targeted by nAbs and discuss the implications for informed vaccine design.
Keywords: Hepatitis C virus; glycoprotein; nAbs; neutralization epitope; neutralizing antibodies; vaccine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Structure-Based Design of Hepatitis C Virus E2 Glycoprotein Improves Serum Binding and Cross-Neutralization.J Virol. 2020 Oct 27;94(22):e00704-20. doi: 10.1128/JVI.00704-20. Print 2020 Oct 27. J Virol. 2020. PMID: 32878891 Free PMC article.
-
Native Folding of a Recombinant gpE1/gpE2 Heterodimer Vaccine Antigen from a Precursor Protein Fused with Fc IgG.J Virol. 2016 Dec 16;91(1):e01552-16. doi: 10.1128/JVI.01552-16. Print 2017 Jan 1. J Virol. 2016. PMID: 27795422 Free PMC article.
-
Structural flexibility of a conserved antigenic region in hepatitis C virus glycoprotein E2 recognized by broadly neutralizing antibodies.J Virol. 2015 Feb;89(4):2170-81. doi: 10.1128/JVI.02190-14. Epub 2014 Dec 3. J Virol. 2015. PMID: 25473061 Free PMC article.
-
Structural perspectives on HCV humoral immune evasion mechanisms.Curr Opin Virol. 2021 Aug;49:92-101. doi: 10.1016/j.coviro.2021.05.002. Epub 2021 Jun 3. Curr Opin Virol. 2021. PMID: 34091143 Free PMC article. Review.
-
Virus-neutralizing antibodies to hepatitis C virus.J Viral Hepat. 2013 Jun;20(6):369-76. doi: 10.1111/jvh.12094. Epub 2013 Apr 4. J Viral Hepat. 2013. PMID: 23647953 Review.
Cited by
-
From Structural Studies to HCV Vaccine Design.Viruses. 2021 May 4;13(5):833. doi: 10.3390/v13050833. Viruses. 2021. PMID: 34064532 Free PMC article. Review.
-
How glycobiology can help us treat and beat the COVID-19 pandemic.J Biol Chem. 2021 Jan-Jun;296:100375. doi: 10.1016/j.jbc.2021.100375. Epub 2021 Feb 4. J Biol Chem. 2021. PMID: 33548227 Free PMC article. Review.
-
Prokaryote- and Eukaryote-Based Expression Systems: Advances in Post-Pandemic Viral Antigen Production for Vaccines.Int J Mol Sci. 2024 Nov 7;25(22):11979. doi: 10.3390/ijms252211979. Int J Mol Sci. 2024. PMID: 39596049 Free PMC article. Review.
-
Analysis of antibodies from HCV elite neutralizers identifies genetic determinants of broad neutralization.Immunity. 2022 Feb 8;55(2):341-354.e7. doi: 10.1016/j.immuni.2021.12.003. Epub 2022 Jan 5. Immunity. 2022. PMID: 34990590 Free PMC article.
-
Sustained Long-Term Decline in Anti-HCV Neutralizing Antibodies in HIV/HCV-Coinfected Patients Five Years after HCV Therapy: A Retrospective Study.Pharmaceuticals (Basel). 2024 Aug 30;17(9):1152. doi: 10.3390/ph17091152. Pharmaceuticals (Basel). 2024. PMID: 39338314 Free PMC article.
References
-
- World Health Organization . Global Hepatitis Report. World Health Organization; Geneva, Switzerland: 2017.
-
- Enkelmann J., Gassowski M., Nielsen S., Wenz B., Ross S., Marcus U., Bremer V., Zimmermann R., DRUCK Study Group High prevalence of hepatitis C virus infection and low level of awareness among people who recently started injecting drugs in a cross-sectional study in Germany, 2011–2014: Missed opportunities for hepatitis C testing. Harm. Reduct. J. 2020;17:1–10. doi: 10.1186/s12954-019-0338-y. - DOI - PMC - PubMed
-
- Reig M., Marino Z., Perello C., Inarrairaegui M., Ribeiro A., Lens S., Diaz A., Vilana R., Darnell A., Varela M., et al. Unexpected high rate of early tumor recurrence in patients with HCV-related HCC undergoing interferon-free therapy. J. Hepatol. 2016;65:719–726. doi: 10.1016/j.jhep.2016.04.008. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

