Osteoclasts and Microgravity
- PMID: 32947946
- PMCID: PMC7555718
- DOI: 10.3390/life10090207
Osteoclasts and Microgravity
Abstract
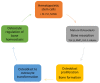
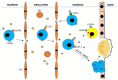
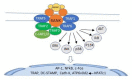
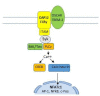
Astronauts are at risk of losing 1.0% to 1.5% of their bone mass for every month they spend in space despite their adherence to diets and exercise regimens designed to protect their musculoskeletal systems. This loss is the result of microgravity-related impairment of osteocyte and osteoblast function and the consequent upregulation of osteoclast-mediated bone resorption. This review describes the ontogeny of osteoclast hematopoietic stem cells and the contributions macrophage colony stimulating factor, receptor activator of the nuclear factor-kappa B ligand, and the calcineurin pathways make in osteoclast differentiation and provides details of bone formation, the osteoclast cytoskeleton, the immune regulation of osteoclasts, and osteoclast mechanotransduction on Earth, in space, and under conditions of simulated microgravity. The article discusses the need to better understand how osteoclasts are able to function in zero gravity and reviews current and prospective therapies that may be used to treat osteoclast-mediated bone disease.
Keywords: M-CSF; RANKL; bone; cytokines; microgravity; osteoblasts; osteoclasts; osteocytes; spaceflight.
Conflict of interest statement
The author declares no conflict of interest.
Figures



References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

