Suppression of Oxidative Stress as Potential Therapeutic Approach for Normal Tension Glaucoma
- PMID: 32947996
- PMCID: PMC7554707
- DOI: 10.3390/antiox9090874
Suppression of Oxidative Stress as Potential Therapeutic Approach for Normal Tension Glaucoma
Abstract
Glaucoma is a neurodegenerative disease of the eye, which involves degeneration of retinal ganglion cells (RGCs): the output neurons of the retina to the brain, which with their axons comprise the optic nerve. Recent studies have shown the possible involvement of oxidative stress in the pathogenesis of glaucoma, especially in the subtype of normal tension glaucoma. Basic experiments utilizing rodent and primate models of glaucoma revealed that antioxidants protect RGCs under various pathological conditions including glutamate neurotoxicity and optic nerve injury. These results suggested that existing drugs and food factors may be useful for prevention and hence therapy of glaucoma. In this review, we highlight some therapeutic candidates, particularly those with antioxidant properties, and discuss the therapeutic potential of RGC protection by modulating gene expressions that prevent and ameliorate glaucoma.
Keywords: ASK1; drug repositioning; food factor; glaucoma; glutamate transporters; marmoset; neuroprotection; optic nerve; oxidative stress; retinal ganglion cell.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
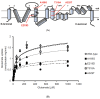
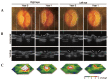

References
-
- Honda S., Namekata K., Kimura A., Guo X., Harada C., Murakami A., Matsuda A., Harada T. Survival of alpha and intrinsically photosensitive retinal ganglion cells in NMDA-induced neurotoxicity and a mouse model of normal tension glaucoma. Investig. Ophthalmol. Vis. Sci. 2019;60:3696–3707. doi: 10.1167/iovs.19-27145. - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

