The Role of Extracellular Proteases in Tumor Progression and the Development of Innovative Metal Ion Chelators that Inhibit their Activity
- PMID: 32948029
- PMCID: PMC7555822
- DOI: 10.3390/ijms21186805
The Role of Extracellular Proteases in Tumor Progression and the Development of Innovative Metal Ion Chelators that Inhibit their Activity
Abstract
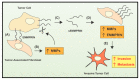
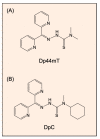
The crucial role of extracellular proteases in cancer progression is well-known, especially in relation to the promotion of cell invasion through extracellular matrix remodeling. This also occurs by the ability of extracellular proteases to induce the shedding of transmembrane proteins at the plasma membrane surface or within extracellular vesicles. This process results in the regulation of key signaling pathways by the modulation of kinases, e.g., the epidermal growth factor receptor (EGFR). Considering their regulatory roles in cancer, therapeutics targeting various extracellular proteases have been discovered. These include the metal-binding agents di-2-pyridylketone 4,4-dimethyl-3-thiosemicarbazone (Dp44mT) and di-2-pyridylketone-4-cyclohexyl-4-methyl-3-thiosemicarbazone (DpC), which increase c-MET degradation by multiple mechanisms. Both the direct and indirect inhibition of protease expression and activity can be achieved through metal ion depletion. Considering direct mechanisms, chelators can bind zinc(II) that plays a catalytic role in enzyme activity. In terms of indirect mechanisms, Dp44mT and DpC potently suppress the expression of the kallikrein-related peptidase-a prostate-specific antigen-in prostate cancer cells. The mechanism of this activity involves promotion of the degradation of the androgen receptor. Additional suppressive mechanisms of Dp44mT and DpC on matrix metalloproteases (MMPs) relate to their ability to up-regulate the metastasis suppressors N-myc downstream regulated gene-1 (NDRG1) and NDRG2, which down-regulate MMPs that are crucial for cancer cell invasion.
Keywords: Keywords: cancer therapeutics; matrix metalloproteases; prostate specific antigen; thiosemicarbazones.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
The Metastasis Suppressor, N-MYC Downstream-regulated Gene-1 (NDRG1), Down-regulates the ErbB Family of Receptors to Inhibit Downstream Oncogenic Signaling Pathways.J Biol Chem. 2016 Jan 15;291(3):1029-52. doi: 10.1074/jbc.M115.689653. Epub 2015 Nov 3. J Biol Chem. 2016. PMID: 26534963 Free PMC article.
-
Thiosemicarbazones suppress expression of the c-Met oncogene by mechanisms involving lysosomal degradation and intracellular shedding.J Biol Chem. 2020 Jan 10;295(2):481-503. doi: 10.1074/jbc.RA119.011341. Epub 2019 Nov 19. J Biol Chem. 2020. PMID: 31744884 Free PMC article.
-
Methemoglobin formation by triapine, di-2-pyridylketone-4,4-dimethyl-3-thiosemicarbazone (Dp44mT), and other anticancer thiosemicarbazones: identification of novel thiosemicarbazones and therapeutics that prevent this effect.Mol Pharmacol. 2012 Jul;82(1):105-14. doi: 10.1124/mol.112.078964. Epub 2012 Apr 16. Mol Pharmacol. 2012. PMID: 22508546
-
Innovative therapies for neuroblastoma: The surprisingly potent role of iron chelation in up-regulating metastasis and tumor suppressors and down-regulating the key oncogene, N-myc.Pharmacol Res. 2021 Nov;173:105889. doi: 10.1016/j.phrs.2021.105889. Epub 2021 Sep 16. Pharmacol Res. 2021. PMID: 34536548 Review.
-
Matrix metalloproteinase inhibitors.Invest New Drugs. 1997;15(1):61-75. doi: 10.1023/a:1005722729132. Invest New Drugs. 1997. PMID: 9195290 Review.
Cited by
-
Investigating isoform switching in RHBDF2 and its role in neoplastic growth in breast cancer.PeerJ. 2022 Nov 25;10:e14124. doi: 10.7717/peerj.14124. eCollection 2022. PeerJ. 2022. PMID: 36452073 Free PMC article.
-
Biology of the Extracellular Proteasome.Biomolecules. 2022 Apr 21;12(5):619. doi: 10.3390/biom12050619. Biomolecules. 2022. PMID: 35625547 Free PMC article. Review.
-
Increasing the odds: antibody-mediated delivery of two distinct immunogenic T-cell epitopes with one antibody.Oncoimmunology. 2025 Dec;14(1):2508050. doi: 10.1080/2162402X.2025.2508050. Epub 2025 May 27. Oncoimmunology. 2025. PMID: 40426019 Free PMC article.
-
PERRC: Protease Engineering with Reactant Residence Time Control.bioRxiv [Preprint]. 2025 Mar 4:2025.03.02.641063. doi: 10.1101/2025.03.02.641063. bioRxiv. 2025. Update in: ACS Synth Biol. 2025 Jun 20;14(6):2241-2253. doi: 10.1021/acssynbio.5c00154. PMID: 40093119 Free PMC article. Updated. Preprint.
-
PhAc-ALGP-Dox, a Novel Anticancer Prodrug with Targeted Activation and Improved Therapeutic Index.Mol Cancer Ther. 2022 Apr 1;21(4):568-581. doi: 10.1158/1535-7163.MCT-21-0518. Mol Cancer Ther. 2022. PMID: 35149549 Free PMC article.
References
-
- Moali C., Hulmes D.J. Extracellular and cell surface proteases in wound healing: New players are still emerging. Eur. J. Dermatol. 2009;19:552–564. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

