Biased Opioid Ligands
- PMID: 32948048
- PMCID: PMC7570672
- DOI: 10.3390/molecules25184257
Biased Opioid Ligands
Abstract
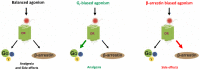
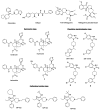
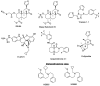
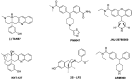
Achieving effective pain management is one of the major challenges associated with modern day medicine. Opioids, such as morphine, have been the reference treatment for moderate to severe acute pain not excluding chronic pain modalities. Opioids act through the opioid receptors, the family of G-protein coupled receptors (GPCRs) that mediate pain relief through both the central and peripheral nervous systems. Four types of opioid receptors have been described, including the μ-opioid receptor (MOR), κ-opioid receptor (KOR), δ-opioid receptor (DOR), and the nociceptin opioid peptide receptor (NOP receptor). Despite the proven success of opioids in treating pain, there are still some inherent limitations. All clinically approved MOR analgesics are associated with adverse effects, which include tolerance, dependence, addiction, constipation, and respiratory depression. On the other hand, KOR selective analgesics have found limited clinical utility because they cause sedation, anxiety, dysphoria, and hallucinations. DOR agonists have also been investigated but they have a tendency to cause convulsions. Ligands targeting NOP receptor have been reported in the preclinical literature to be useful as spinal analgesics and as entities against substance abuse disorders while mixed MOR/NOP receptor agonists are useful as analgesics. Ultimately, the goal of opioid-related drug development has always been to design and synthesize derivatives that are equally or more potent than morphine but most importantly are devoid of the dangerous residual side effects and abuse potential. One proposed strategy is to take advantage of biased agonism, in which distinct downstream pathways can be activated by different molecules working through the exact same receptor. It has been proposed that ligands not recruiting β-arrestin 2 or showing a preference for activating a specific G-protein mediated signal transduction pathway will function as safer analgesic across all opioid subtypes. This review will focus on the design and the pharmacological outcomes of biased ligands at the opioid receptors, aiming at achieving functional selectivity.
Keywords: G-protein bias; analgesia; arrestin recruitment; mitragynine; opioid receptors; respiration.
Conflict of interest statement
S.M. is the cofounder of Sparian Inc. and has patents related to mitragyna alkaloids and its derivatives. The other authors report no other conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

