Lower versus higher diagnostic criteria for the detection of gestational diabetes for reducing maternal and perinatal morbidity: study protocol for the GEMS randomised trial
- PMID: 32948138
- PMCID: PMC7501633
- DOI: 10.1186/s12884-020-03252-9
Lower versus higher diagnostic criteria for the detection of gestational diabetes for reducing maternal and perinatal morbidity: study protocol for the GEMS randomised trial
Abstract
Background: Gestational diabetes mellitus (GDM) has lifelong implications for the woman and her infant. Treatment reduces adverse maternal and perinatal outcomes although uncertainty remains about the optimal diagnostic criteria. The GEMS Trial aims to assess whether detection and treatment of women with GDM using the lower International Association of Diabetes in Pregnancy Study Groups diagnostic criteria compared with the higher criteria recommended in New Zealand reduces infant morbidity without increasing maternal morbidity.
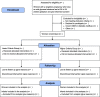
Methods: GEMS is a multicentre, randomised trial. Women with a singleton pregnancy at 24 to 34 weeks' gestation are eligible who give written informed consent. Women are randomly allocated to the Lower Criteria Group or the Higher Criteria Group. Women with a normal OGTT by their allocated criteria receive routine care (Higher criteria: fasting plasma glucose < 5.5 mmol/L, AND 2 hour < 9.0 mmol/L; Lower criteria: fasting plasma glucose < 5.1 mmol/L, AND 1 hour < 10.0 mmol/L, AND 2 hour < 8.5 mmol/l). Women with GDM on OGTT by their allocated criteria receive standard care for GDM (Higher criteria: fasting plasma glucose ≥ 5.5 mmol/L, OR 2 hour ≥ 9.0 mmol/L; Lower criteria: fasting plasma glucose ≥ 5.1 mmol/L, OR 1 hour ≥ 10.0 mmol/L, OR 2 hour ≥ 8.5 mmol/L). The primary outcome is large for gestational age (birth weight > 90th centile). Secondary outcomes for the infant include a composite of serious outcomes, gestational age, anthropometry, Apgar score < 4 at 5 minutes, lung disease, use of respiratory support, hypoglycaemia, hyperbilirubinaemia, infection, and encephalopathy; and for the woman, a composite of serious outcomes, preeclampsia, induction of labour, mode of birth, weight gain, postpartum haemorrhage and infectious morbidity. A study with 4,158 women will detect an absolute difference of 2.9% in the proportion of large for gestational age infants from 10.0% using the lower criteria to 12.9% with the higher criteria.
Discussion: The GEMS Trial will provide high-level evidence relevant for clinical practice. If use of the lower diagnostic criteria results in significantly fewer large for gestational age infants and/or improves maternal and perinatal outcomes these criteria should be recommended for diagnosis of gestational diabetes.
Trial registration: Australian New Zealand Clinical Trials Registry registration number ACTRN12615000290594 . Date registered: 27th March 2015.
Keywords: Gestational diabetes mellitus; diagnostic threshold; large for gestational age; randomised trial.
Conflict of interest statement
The authors declare they have no competing interests.
Figures
References
-
- Sacks D, Hadden D, Maresh M, Deerochanawong C, Dyer A, Metzger B, Lowe L, Coustan D, Hod M, Oats J, Persson B, Trimble E, HAPO Study Cooperative Research Group Frequency of gestational diabetes mellitus at collaborating centers based on IADPSG consensus panel recommended criteria. Diabetes Care. 2012;35(3):526–28. doi: 10.2337/dc11-1641. - DOI - PMC - PubMed
-
- Dodd JM, Crowther CA, Antoniou G, Baghurst P, Robinson JS. Screening for gestational diabetes: the effect of varying blood glucose definitions in the prediction of adverse maternal and infant health outcomes. Aust N Z J Obstet Gynaecol. 2007;47(4):307–12. doi: 10.1111/j.1479-828X.2007.00743.x. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical