Facilitating islet transplantation using a three-step approach with mesenchymal stem cells, encapsulation, and pulsed focused ultrasound
- PMID: 32948247
- PMCID: PMC7501701
- DOI: 10.1186/s13287-020-01897-z
Facilitating islet transplantation using a three-step approach with mesenchymal stem cells, encapsulation, and pulsed focused ultrasound
Erratum in
-
Correction: Facilitating islet transplantation using a three-step approach with mesenchymal stem cells, encapsulation, and pulsed focused ultrasound.Stem Cell Res Ther. 2022 Dec 20;13(1):526. doi: 10.1186/s13287-022-03210-6. Stem Cell Res Ther. 2022. PMID: 36536426 Free PMC article. No abstract available.
Abstract
Background: The aim of this study was to examine the effect of a three-step approach that utilizes the application of adipose tissue-derived mesenchymal stem cells (AD-MSCs), encapsulation, and pulsed focused ultrasound (pFUS) to help the engraftment and function of transplanted islets.
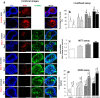
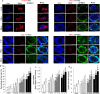
Methods: In step 1, islets were co-cultured with AD-MSCs to form a coating of AD-MSCs on islets: here, AD-MSCs had a cytoprotective effect on islets; in step 2, islets coated with AD-MSCs were conformally encapsulated in a thin layer of alginate using a co-axial air-flow method: here, the capsule enabled AD-MSCs to be in close proximity to islets; in step 3, encapsulated islets coated with AD-MSCs were treated with pFUS: here, pFUS enhanced the secretion of insulin from islets as well as stimulated the cytoprotective effect of AD-MSCs.
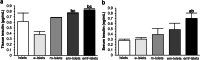
Results: Our approach was shown to prevent islet death and preserve islet functionality in vitro. When 175 syngeneic encapsulated islets coated with AD-MSCs were transplanted beneath the kidney capsule of diabetic mice, and then followed every 3 days with pFUS treatment until day 12 post-transplantation, we saw a significant improvement in islet function with diabetic animals re-establishing glycemic control over the course of our study (i.e., 30 days). In addition, our approach was able to enhance islet engraftment by facilitating their revascularization and reducing inflammation.
Conclusions: This study demonstrates that our clinically translatable three-step approach is able to improve the function and viability of transplanted islets.
Keywords: Diabetes; Encapsulation; Islets transplantation; Mesenchymal stem cells; Pulsed focused ultrasound.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




Similar articles
-
Co-transplantation of islets with mesenchymal stem cells in microcapsules demonstrates graft outcome can be improved in an isolated-graft model of islet transplantation in mice.Cytotherapy. 2013 Feb;15(2):192-200. doi: 10.1016/j.jcyt.2012.10.018. Cytotherapy. 2013. PMID: 23321331
-
Adipose tissue-derived mesenchymal stem cells rescue the function of islets transplanted in sub-therapeutic numbers via their angiogenic properties.Cell Tissue Res. 2019 Jun;376(3):353-364. doi: 10.1007/s00441-019-02997-w. Epub 2019 Feb 1. Cell Tissue Res. 2019. PMID: 30707291 Free PMC article.
-
Improving the Function and Engraftment of Transplanted Pancreatic Islets Using Pulsed Focused Ultrasound Therapy.Sci Rep. 2019 Sep 16;9(1):13416. doi: 10.1038/s41598-019-49933-0. Sci Rep. 2019. PMID: 31527773 Free PMC article.
-
Using Mesenchymal Stromal Cells in Islet Transplantation.Stem Cells Transl Med. 2018 Aug;7(8):559-563. doi: 10.1002/sctm.18-0033. Epub 2018 May 11. Stem Cells Transl Med. 2018. PMID: 29749717 Free PMC article. Review.
-
Protecting islet functional viability using mesenchymal stromal cells.Stem Cells Transl Med. 2021 May;10(5):674-680. doi: 10.1002/sctm.20-0466. Epub 2021 Feb 5. Stem Cells Transl Med. 2021. PMID: 33544449 Free PMC article. Review.
Cited by
-
Importance of multiple endocrine cell types in islet organoids for type 1 diabetes treatment.Transl Res. 2022 Dec;250:68-83. doi: 10.1016/j.trsl.2022.06.014. Epub 2022 Jun 28. Transl Res. 2022. PMID: 35772687 Free PMC article. Review.
-
Immunoprotection Strategies in β-Cell Replacement Therapy: A Closer Look at Porcine Islet Xenotransplantation.Adv Sci (Weinh). 2024 Aug;11(31):e2401385. doi: 10.1002/advs.202401385. Epub 2024 Jun 17. Adv Sci (Weinh). 2024. PMID: 38884159 Free PMC article. Review.
-
Strategy for Clinical Setting of Co-transplantation of Mesenchymal Stem Cells and Pancreatic Islets.Cell Transplant. 2024 Jan-Dec;33:9636897241259433. doi: 10.1177/09636897241259433. Cell Transplant. 2024. PMID: 38877672 Free PMC article. Review.
-
Shape-recovery of implanted shape-memory devices remotely triggered via image-guided ultrasound heating.Nat Commun. 2024 Feb 6;15(1):1123. doi: 10.1038/s41467-024-45437-2. Nat Commun. 2024. PMID: 38321028 Free PMC article.
-
Mouse models and human islet transplantation sites for intravital imaging.Front Endocrinol (Lausanne). 2022 Oct 5;13:992540. doi: 10.3389/fendo.2022.992540. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 36277698 Free PMC article. Review.
References
-
- S ELA, Mager I, Breakefield XO, Wood MJ. Extracellular vesicles: biology and emerging therapeutic opportunities. Nat Rev Drug Discov 2013;12(5):347–357. - PubMed
-
- Aguayo-Mazzucato C, Bonner-Weir S. Stem cell therapy for type 1 diabetes mellitus. Nat Rev Endocrinol. 2010;6(3):139. - PubMed
-
- Ryan EA, Lakey JR, Rajotte RV, Korbutt GS, Kin T, Imes S, et al. Clinical outcomes and insulin secretion after islet transplantation with the Edmonton protocol. Diabetes. 2001;50(4):710–719. - PubMed
-
- Gillies M, Mandel T. The evolution of function and response to arginine challenge and pregnancy of portally and systemically placed islet cell grafts in streptozotocin diabetic mice. Metabolism. 1990;39(12):1253–1258. - PubMed
-
- Van Der Windt DJ, Bottino R, Casu A, Campanile N, Cooper DK. Rapid loss of intraportally transplanted islets: an overview of pathophysiology and preventive strategies. Xenotransplantation. 2007;14(4):288–297. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

