Early-stage bilayer tissue-engineered skin substitute formed by adult skin progenitor cells produces an improved skin structure in vivo
- PMID: 32948249
- PMCID: PMC7501683
- DOI: 10.1186/s13287-020-01924-z
Early-stage bilayer tissue-engineered skin substitute formed by adult skin progenitor cells produces an improved skin structure in vivo
Abstract
Background: In recent years, significant progress has been made in developing highly complex tissue-engineered skin substitutes (TESSs) for wound healing. However, the lack of skin appendages, such as hair follicles and sweat glands, and the time required, are two major limitations that hinder its broad application in the clinic. Therefore, it is necessary to develop a competent TESS in a short time to meet the needs for clinical applications.
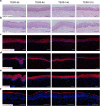
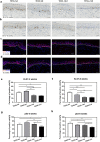
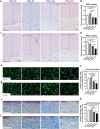
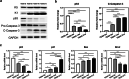
Methods: Adult scalp dermal progenitor cells and epidermal stem cells together with type I collagen as a scaffold material were used to reconstitute bilayer TESSs in vitro. TESSs at 4 different culture times (5, 9, 14, and 21 days) were collected and then grafted onto full-thickness wounds created in the dorsal skin of athymic nude/nude mice. The skin specimens formed from grafted TESSs were collected 4 and 8 weeks later and then evaluated for their structure, cell organization, differentiation status, vascularization, and formation of appendages by histological analysis, immunohistochemistry, and immunofluorescent staining.
Results: Early-stage bilayer TESSs after transplantation had a better efficiency of grafting. A normal structure of stratified epidermis containing multiple differentiated layers of keratinocytes was formed in all grafts from both early-stage and late-stage TESSs, but higher levels of the proliferation marker Ki-67 and the epidermal progenitor marker p63 were found in the epidermis formed from early-stage TESSs. Interestingly, the transplantation of early-stage TESSs produced a thicker dermis that contained more vimentin- and CD31-positive cells, and importantly, hair follicle formation was only observed in the skin grafted from early-stage TESSs. Finally, early-stage TESSs expressed high levels of p63 but had low expression levels of genes involved in the activation of the apoptotic pathway compared to the late-stage TESSs in vitro.
Conclusions: Early-stage bilayer TESSs reconstituted from skin progenitor cells contained more competent cells with less activation of the apoptotic pathway and produced a better skin structure, including hair follicles associated with sebaceous glands, after transplantation, which should potentially provide better wound healing when applied in the clinic in the future.
Keywords: Hair follicle regeneration; Skin progenitor cell; Skin regeneration; Tissue-engineered skin; p63.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


Similar articles
-
[Experimental study on repairing full-thickness cutaneous deficiency with tissue engineered skin].Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2008 Feb;22(2):196-201. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2008. PMID: 18365618 Chinese.
-
Dermal papilla cells improve the wound healing process and generate hair bud-like structures in grafted skin substitutes using hair follicle stem cells.Stem Cells Transl Med. 2014 Oct;3(10):1209-19. doi: 10.5966/sctm.2013-0217. Epub 2014 Aug 26. Stem Cells Transl Med. 2014. PMID: 25161315 Free PMC article.
-
Hair Follicle and Sebaceous Gland De Novo Regeneration With Cultured Epidermal Stem Cells and Skin-Derived Precursors.Stem Cells Transl Med. 2016 Dec;5(12):1695-1706. doi: 10.5966/sctm.2015-0397. Epub 2016 Jul 25. Stem Cells Transl Med. 2016. PMID: 27458264 Free PMC article.
-
Epidermolysis Bullosa: A Review of the Tissue-Engineered Skin Substitutes Used to Treat Wounds.Mol Diagn Ther. 2022 Nov;26(6):627-643. doi: 10.1007/s40291-022-00613-2. Epub 2022 Oct 17. Mol Diagn Ther. 2022. PMID: 36251245 Free PMC article. Review.
-
Tissue Engineered Skin and Wound Healing: Current Strategies and Future Directions.Curr Pharm Des. 2017;23(24):3455-3482. doi: 10.2174/1381612823666170526094606. Curr Pharm Des. 2017. PMID: 28552069 Review.
Cited by
-
A review of the current state of natural biomaterials in wound healing applications.Front Bioeng Biotechnol. 2024 Mar 27;12:1309541. doi: 10.3389/fbioe.2024.1309541. eCollection 2024. Front Bioeng Biotechnol. 2024. PMID: 38600945 Free PMC article. Review.
-
Bioengineered Efficacy Models of Skin Disease: Advances in the Last 10 Years.Pharmaceutics. 2022 Jan 28;14(2):319. doi: 10.3390/pharmaceutics14020319. Pharmaceutics. 2022. PMID: 35214050 Free PMC article. Review.
-
Applications of Engineered Skin Tissue for Cosmetic Component and Toxicology Detection.Cell Transplant. 2024 Jan-Dec;33:9636897241235464. doi: 10.1177/09636897241235464. Cell Transplant. 2024. PMID: 38491929 Free PMC article. Review.
-
Accelular nanofibrous bilayer scaffold intrapenetrated with polydopamine network and implemented into a full-thickness wound of a white-pig model affects inflammation and healing process.J Nanobiotechnology. 2023 Mar 7;21(1):80. doi: 10.1186/s12951-023-01822-5. J Nanobiotechnology. 2023. PMID: 36882867 Free PMC article.
-
Establishing a High Throughput Epidermal Spheroid Culture System to Model Keratinocyte Stem Cell Plasticity.J Vis Exp. 2021 Jan 30;(167):10.3791/62182. doi: 10.3791/62182. J Vis Exp. 2021. PMID: 33586700 Free PMC article.
References
-
- Sun BK, Siprashvili Z, Khavari PA. Advances in skin grafting and treatment of cutaneous wounds. Science. 2014;346:941–945. - PubMed
-
- Peppas N, Langer R. New challenges in biomaterials. Science. 1994;263:1715–1720. - PubMed
-
- Gallico GG, O’Connor NE, Compton CC, Kehinde O, Green H. Permanent coverage of large burn wounds with autologous cultured human epithelium. N Engl J Med. 1984;311:448–451. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

