Antibody-like proteins that capture and neutralize SARS-CoV-2
- PMID: 32948512
- PMCID: PMC7556756
- DOI: 10.1126/sciadv.abd3916
Antibody-like proteins that capture and neutralize SARS-CoV-2
Abstract
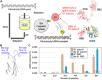
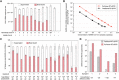
To combat severe acute respiratory syndrome-related coronavirus 2 (SARS-CoV-2) and any unknown emerging pathogens in the future, the development of a rapid and effective method to generate high-affinity antibodies or antibody-like proteins is of critical importance. We here report high-speed in vitro selection of multiple high-affinity antibody-like proteins against various targets including the SARS-CoV-2 spike protein. The sequences of monobodies against the SARS-CoV-2 spike protein were successfully procured within only 4 days. Furthermore, the obtained monobody efficiently captured SARS-CoV-2 particles from the nasal swab samples of patients and exhibited a high neutralizing activity against SARS-CoV-2 infection (half-maximal inhibitory concentration, 0.5 nanomolar). High-speed in vitro selection of antibody-like proteins is a promising method for rapid development of a detection method for, and of a neutralizing protein against, a virus responsible for an ongoing, and possibly a future, pandemic.
Copyright © 2020 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works. Distributed under a Creative Commons Attribution NonCommercial License 4.0 (CC BY-NC).
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

